JEE Advance - Chemistry (2023 - Paper 2 Online - No. 13)
The mass (in mg) of $\mathbf{S}$ obtained is ________.
[Use molar mass (in $\mathrm{g} \mathrm{mol}^{-1}$ ) : $\mathrm{H}=1, \mathrm{C}=12, \mathrm{~N}=14, \mathrm{Br}=80$ ]
Explanation
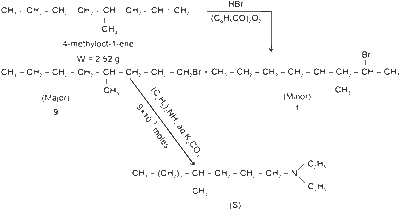
First, calculate the moles of 4-methyloct-1-ene. The molar mass of 4-methyloct-1-ene is 126 g/mol. So, the moles of 4-methyloct-1-ene are :
$$n_{4-\text{methyloct-1-ene}} = \frac{2.52 \, g}{126 \, g/mol} = 0.02 \, mol$$
Given that the combined yield is 50%, the amount of isomeric bromides produced will be half of the initial amount, which is 0.01 mol.
The isomeric bromides are produced in a 9 : 1 ratio, so 90% of the product, or 0.009 mol, is the primary alkyl bromide. This is the compound that reacts further with diethylamine.
The reaction with diethylamine leads to the non-ionic product S with 100% yield, so we will still have 0.009 mol of product S.
Based on the chemical structures provided, the molar mass of S is 199 g/mol.
We can now calculate the mass of product S using the equation :
$$ \text{Mass of S} = \text{moles of S} \times \text{molar mass of S} = 0.009 \, mol \times 199 \, g/mol = 1.791 \, g$$
To convert this into milligrams, we multiply by 1000, yielding a mass of S obtained equal to 1791 mg.
Comments (0)


