JEE Advance - Chemistry (2023 - Paper 2 Online - No. 10)
Consider the following molecules: $\mathrm{Br}_3 \mathrm{O}_8, \mathrm{~F}_2 \mathrm{O}, \mathrm{H}_2 \mathrm{S}_4 \mathrm{O}_6, \mathrm{H}_2 \mathrm{S}_5 \mathrm{O}_6$, and $\mathrm{C}_3 \mathrm{O}_2$. Count the number of atoms existing in their zero oxidation state in each molecule.
Their sum is _______.
Their sum is _______.
Answer
6
Explanation
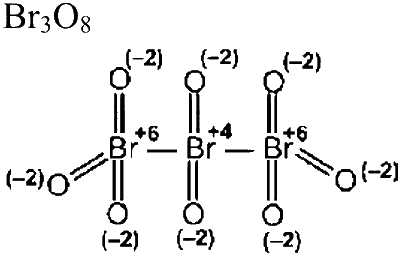
Number of atoms with zero oxidation state $=0$
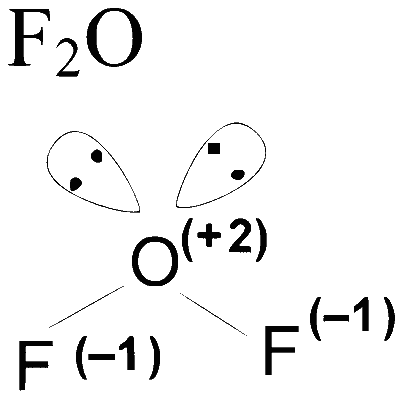
Number of atom with zero oxidation state $=0$
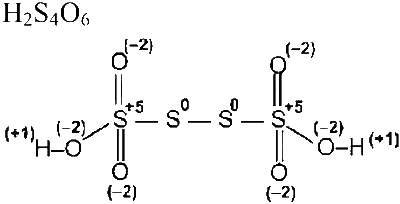
Number of atoms where zero oxidation state $=2$
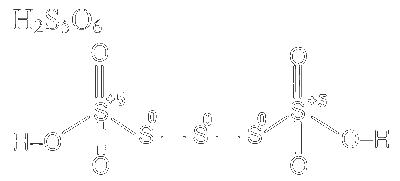
Number of atoms where zero oxidation state $=3$
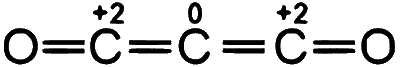
Number of atoms with zero oxidation state $=1$
$$ \therefore $$ Total atom with zero oxidation number state are 6.
Comments (0)


