JEE Advance - Chemistry (2023 - Paper 2 Online - No. 1)
The correct molecular orbital diagram for $\mathrm{F}_2$ molecule in the ground state is :


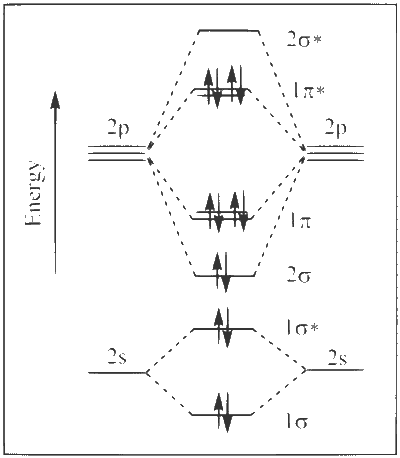

Explanation
(1) Upto 14 electrons, molecular orbital configuration is ->

Nb = No of electrons in bonding molecular orbital
Na $$=$$ No of electrons in anti bonding molecular orbital
Here Na = Anti bonding electron $$=$$ 4 and Nb = 10
(2) After 14 electrons to 20 electrons molecular orbital configuration is ->

Here Na = 10
and Nb = 10
Now from question, in F atom 9 electrons present, so in F2, 9 $$ \times $$ 2 = 18 electrons present.
Molecular orbital configuration of F2(18 electrons) is
$${\sigma _{1{s^2}}}\,\sigma _{1{s^2}}^ * \,{\sigma _{2{s^2}}}\,\sigma _{2{s^2}}^ * \,{\sigma _{2p_z^2}}\,{\pi _{2p_x^2}}\, = \,{\pi _{2p_y^2}}\,\pi _{2p_x^2}^ * \, = \,\pi _{2p_y^2}^ * $$

Nb = No of electrons in bonding molecular orbital
Na $$=$$ No of electrons in anti bonding molecular orbital
Here Na = Anti bonding electron $$=$$ 4 and Nb = 10
(2) After 14 electrons to 20 electrons molecular orbital configuration is ->

Here Na = 10
and Nb = 10
Now from question, in F atom 9 electrons present, so in F2, 9 $$ \times $$ 2 = 18 electrons present.
Molecular orbital configuration of F2(18 electrons) is
$${\sigma _{1{s^2}}}\,\sigma _{1{s^2}}^ * \,{\sigma _{2{s^2}}}\,\sigma _{2{s^2}}^ * \,{\sigma _{2p_z^2}}\,{\pi _{2p_x^2}}\, = \,{\pi _{2p_y^2}}\,\pi _{2p_x^2}^ * \, = \,\pi _{2p_y^2}^ * $$
Comments (0)


