JEE Advance - Chemistry (2021 - Paper 1 Online - No. 7)
The value of standard enthalpy, $$\Delta$$Ho (in kJ mol$$-$$1) for the given reaction is _______.
Answer
166.28
Explanation
For the given reaction,
$$ \mathrm{X}(\mathrm{s}) \rightleftharpoons \mathrm{X}(\mathrm{s})+\mathrm{Z}(\mathrm{g}) $$
We have
$$ \frac{d(\ln K)}{d(1 / T)}=\frac{-\Delta H^{\circ}}{R} $$
And $$ K=\frac{p_{z}}{p^{\circ}} $$
Substituting the value of $$K$$ in Eq. (1), we get
$$ \frac{d\left(\ln \frac{p_{z}}{p^{\circ}}\right)}{d\left(\frac{1}{T}\right)}=\frac{-\Delta H^{\circ}}{R} $$
Or
$$ d\left(\ln \frac{p_{z}}{p^{\circ}}\right)=\frac{-\Delta H^{\circ}}{R} d\left(\frac{1}{T}\right) $$
Integrating both sides of Eq. (2), we get
$$ \ln \frac{p_{z}}{p^{\circ}}=\frac{-\Delta H^{\circ}}{R T}+\ln A $$
Where $$\ln A=$$ Integration constant
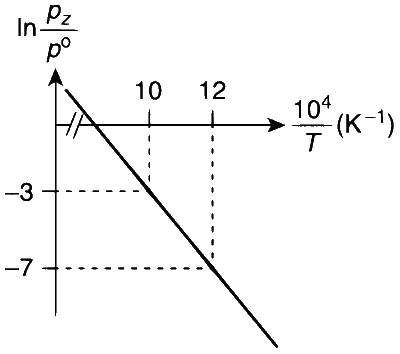
From the given plot of $$\ln \frac{p_{z}}{p^{\circ}} \mathrm{vs} \frac{10^{4}}{T}$$
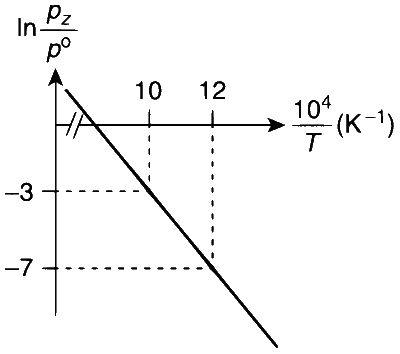
The slope of the plot is
$$ \text { Slope }=\frac{-\Delta H^{\circ}}{R}=\frac{y_{2}-y_{1}}{x_{2}-x_{1}}=\frac{-7-(-3)}{\left(\frac{12}{10^{4}}-\frac{10}{10^{4}}\right)} $$
$$\frac{-\Delta H^{\circ}}{R}=\frac{-4}{2} \times 10^{4}$$
$$\Delta H^{\circ}=-2 \times 10^{4} \times R$$
$$\Delta H^{\circ}=2 \times 8.314 \times 10^{4}$$
$$\Delta H^{\circ}=16.628 \times 10^{4} \mathrm{~J} \mathrm{~mol}^{-1}$$
$$\Delta H^{\circ}=166.28 \mathrm{~kJ} \mathrm{~mol}^{-1}$$
$$ \mathrm{X}(\mathrm{s}) \rightleftharpoons \mathrm{X}(\mathrm{s})+\mathrm{Z}(\mathrm{g}) $$
We have
$$ \frac{d(\ln K)}{d(1 / T)}=\frac{-\Delta H^{\circ}}{R} $$
And $$ K=\frac{p_{z}}{p^{\circ}} $$
Substituting the value of $$K$$ in Eq. (1), we get
$$ \frac{d\left(\ln \frac{p_{z}}{p^{\circ}}\right)}{d\left(\frac{1}{T}\right)}=\frac{-\Delta H^{\circ}}{R} $$
Or
$$ d\left(\ln \frac{p_{z}}{p^{\circ}}\right)=\frac{-\Delta H^{\circ}}{R} d\left(\frac{1}{T}\right) $$
Integrating both sides of Eq. (2), we get
$$ \ln \frac{p_{z}}{p^{\circ}}=\frac{-\Delta H^{\circ}}{R T}+\ln A $$
Where $$\ln A=$$ Integration constant
From the given plot of $$\ln \frac{p_{z}}{p^{\circ}} \mathrm{vs} \frac{10^{4}}{T}$$

The slope of the plot is
$$ \text { Slope }=\frac{-\Delta H^{\circ}}{R}=\frac{y_{2}-y_{1}}{x_{2}-x_{1}}=\frac{-7-(-3)}{\left(\frac{12}{10^{4}}-\frac{10}{10^{4}}\right)} $$
$$\frac{-\Delta H^{\circ}}{R}=\frac{-4}{2} \times 10^{4}$$
$$\Delta H^{\circ}=-2 \times 10^{4} \times R$$
$$\Delta H^{\circ}=2 \times 8.314 \times 10^{4}$$
$$\Delta H^{\circ}=16.628 \times 10^{4} \mathrm{~J} \mathrm{~mol}^{-1}$$
$$\Delta H^{\circ}=166.28 \mathrm{~kJ} \mathrm{~mol}^{-1}$$
Comments (0)