JEE Advance - Chemistry (2021 - Paper 1 Online - No. 4)
The calculated spin only magnetic moments of [
Cr(NH3)6]3+ and [CuF6]3$$-$$ in BM, respectively, are
(Atomic numbers of Cr and Cu are 24 and 29, respectively)
(Atomic numbers of Cr and Cu are 24 and 29, respectively)
3.87 and 2.84
4.90 and 1.73
3.87 and 1.73
4.90 and 2.84
Explanation
$${[Cr{(N{H_3})_6}]^{3 + }}$$
Atomic number of Cr = 24
Electron configuration of Cr = 1s2 2s2 2p6 3s2 3p6 3d5 4s1
Electronic configuration of Cr3+ = 1s2 2s2 2p6 3s2 3p6 3d3 4s0
NH3 is a weak field ligand. The splitting of d-orbital occur.
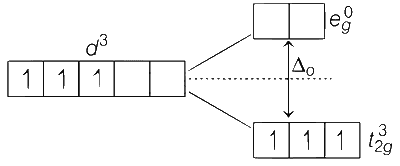
Number of unpaired electrons, n = 3
Magnetic moment (spin only), $${\mu _s} = \sqrt {n(n + 2)} BM$$
$$ = \sqrt {3(3 + 2)} = \sqrt {3 \times 5} = \sqrt {15} = 3.87BM$$
$${[Cu{F_6}]^{3 - }}$$ Atomic number of Cu = 29
Electronic configuration of Cu = 1s2 2s2 2p6 3s2 3p6 3d10 4s1
Electronic configuration of Cu2+ = 1s2 2s2 2p6 3s2 3p6 3d8 4s0
F$$-$$ is a weak field ligand, so splitting of d-orbitals occur as follows:
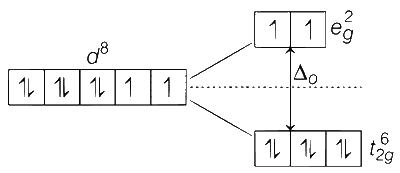
Number of unpaired electrons, n = 2
Spin only magnetic moment, $${\mu _s} = \sqrt {n(n + 2)} BM$$
$$ = \sqrt {2(2 + 2)} = \sqrt {2 \times 4} = 2\sqrt 2 = 2.84BM$$
Atomic number of Cr = 24
Electron configuration of Cr = 1s2 2s2 2p6 3s2 3p6 3d5 4s1
Electronic configuration of Cr3+ = 1s2 2s2 2p6 3s2 3p6 3d3 4s0
NH3 is a weak field ligand. The splitting of d-orbital occur.

Number of unpaired electrons, n = 3
Magnetic moment (spin only), $${\mu _s} = \sqrt {n(n + 2)} BM$$
$$ = \sqrt {3(3 + 2)} = \sqrt {3 \times 5} = \sqrt {15} = 3.87BM$$
$${[Cu{F_6}]^{3 - }}$$ Atomic number of Cu = 29
Electronic configuration of Cu = 1s2 2s2 2p6 3s2 3p6 3d10 4s1
Electronic configuration of Cu2+ = 1s2 2s2 2p6 3s2 3p6 3d8 4s0
F$$-$$ is a weak field ligand, so splitting of d-orbitals occur as follows:

Number of unpaired electrons, n = 2
Spin only magnetic moment, $${\mu _s} = \sqrt {n(n + 2)} BM$$
$$ = \sqrt {2(2 + 2)} = \sqrt {2 \times 4} = 2\sqrt 2 = 2.84BM$$
Comments (0)


