JEE Advance - Chemistry (2021 - Paper 1 Online - No. 19)
The total number of possible isomers for [Pt(NH3)4Cl2]Br2 is ______.
Answer
6
Explanation
The given complex [Pt(NH3)4Cl2]Br2 has three ionisation isomers and each of them has two geometrical isomers.
Three ionisation isomers are
(i) [Pt(NH3)4Cl2]Br2
(ii) [Pt(NH3)4ClBr]BrCl
(iii) [Pt(NH3)4Br2]Cl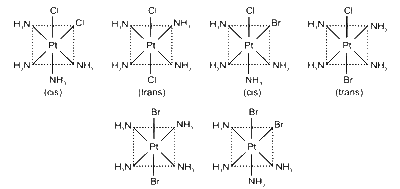
Three ionisation isomers are
(i) [Pt(NH3)4Cl2]Br2
(ii) [Pt(NH3)4ClBr]BrCl
(iii) [Pt(NH3)4Br2]Cl
Ionisation isomers are compounds having same molecular formula but have different counter ions.
Each isomer shown above also possess two more geometrical isomers. Geometrical isomers are the compounds having different arrangement of atoms in space but same molecular formula.
The geometrical isomers are :

Comments (0)


