JEE Advance - Chemistry (2021 - Paper 1 Online - No. 17)
Explanation
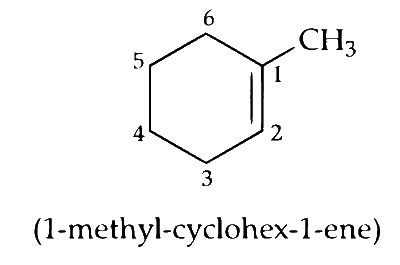
(i) Mono-bromination of substituted methyl on 1-methylcyclohex-1-ene.
Only one stereoisomer possible as compound (1) is optically active.
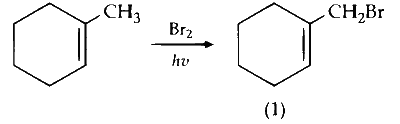
(ii) Mono-bromination of allylic carbon (C-3) :
C-3 is optically active on bromine substitution and gives two optically active isomers.
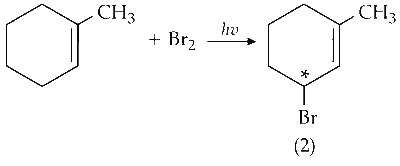
(iii) Mono-bromination of C-4 carbon :
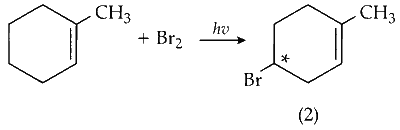
C-4 is optically active on mono-bromination and gives two optically active isomers.
(iv) Mono-bromination of C-5 carbon :
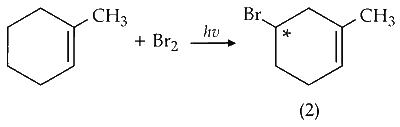
C-5 is optically active on mono-bromination and gives two optically active isomers.
(v) Mono-bromination of C-6 carbon :
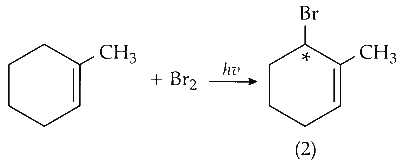
C-6 is optically active on mono-bromination and gives two optically active isomers.
(vi) Mono-bromination of C-1 carbon :
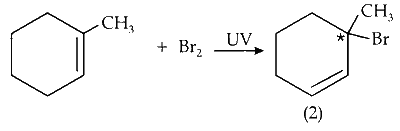
C-1 is optically active on mono-bromination and gives two optically active isomers.
(vii) Mono-bromination of C-2 carbon :
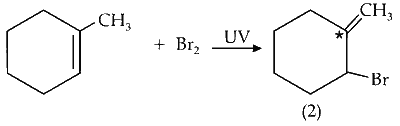
C-2 is optically active on mono-bromination and gives two optically active isomers.
So, maximum number of possible isomers are 13.
Comments (0)


