JEE Advance - Chemistry (2021 - Paper 1 Online - No. 16)
A mixture of two salts is used to prepare a solution S, which gives the following results :

The correct option(s) for the salt mixture is (are)

The correct option(s) for the salt mixture is (are)
Pb(NO3)2 and Zn(NO3)2
Pb(NO3)2 and Bi(NO3)3
AgNO3 and Bi(NO3)3
Pb(NO3)2 and Hg(NO3)2
Explanation
Pb(NO3)2, Bi(NO3)3 and Zn(NO3)2 give white precipitate
with dil. NaOH and dil. HCl. Complete reactions are as follows:
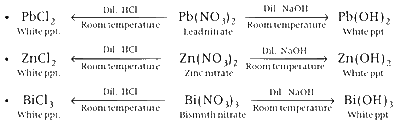
Whereas, AgNO3 and Hg(NO3)3 give ppt. of different colours.
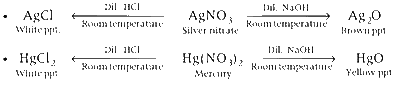

Whereas, AgNO3 and Hg(NO3)3 give ppt. of different colours.
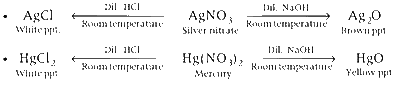
Comments (0)


