JEE Advance - Chemistry (2021 - Paper 1 Online - No. 15)
The correct statement(s) related to the metal extraction processes is (are) :
A mixture of PbS and PbO undergoes self-reduction to produce Pb and SO2
In the extraction process of copper from copper pyrites, silica is added to produce copper silicate.
Partial oxidation of sulphide ore of copper by roasting, followed by self-reduction produces blister copper.
In cyanide process, zinc powder is utilized to precipitate gold from Na[Au(CN)2]
Explanation
Statements (a), (c) and (d) are correct, whereas statement (b) is incorrect. All statements are explained as follows :
(a) A mixture PbO and PbS reacts as follows:
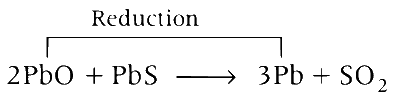
This reaction is known as self-reduction.
(b) Silica is added to remove impurities of Fe in the form of slag, FeSiO3 .
(c) CuFeS2 are in partially oxidised first by roasting and then self-reduction of Cu takes place to produce blister copper.
(d) Zn powder reacts with Na[Au(CN)2 ] to form gold. This process is called cyanide process.
2Zn + 4Na[Au(CN)2] → 2Na2[Zn(CN)4]+ 4Au
Comments (0)


