JEE Advance - Chemistry (2021 - Paper 1 Online - No. 14)
(p : pressure, V : volume, T : temperature, H : enthalpy, S : entropy)
Explanation
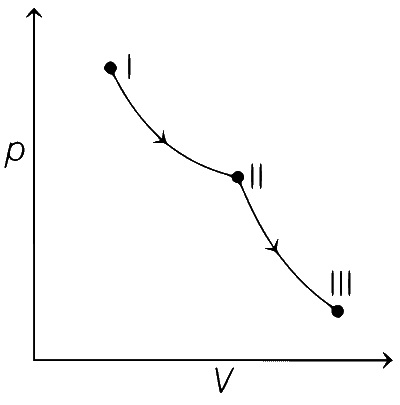
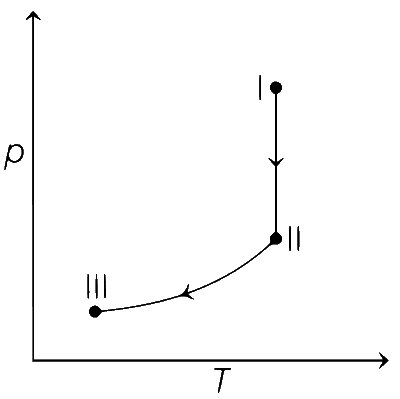
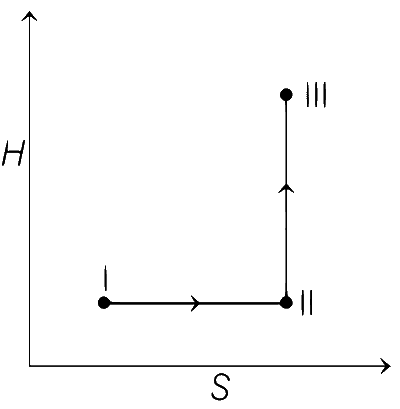
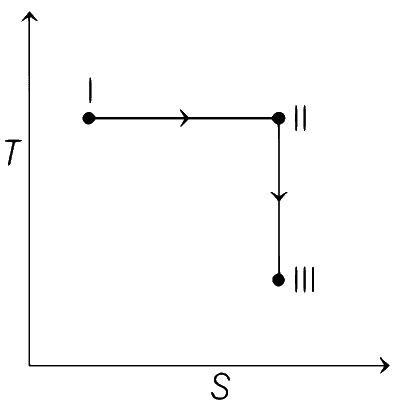
From state I to II Reversible isothermal expansion takes place. So, following changes take place,
● pressure decreases.
● Volume increases.
● Temperature remains constant.
● Enthalpy, H remains constant.
● Entropy, S for expansion increases.
So, all options follows the above mentioned conditions, so all graphs are correct for state I and II.
From state II to III Reversible adiabatic expansion takes place. So, following changes take place.
● pressure decreases.
● Volume increases.
● Temperature decreases.
● Enthalpy, H decreases.
● Entropy, S remains constant.
● H increases instead of decreasing, so only option (c) is
incorrect.
All other options, i.e., (a), (b) and (d) follows the above mentioned conditions.
Therefore, correct graphical representations are (a), (b) and (d).
Comments (0)


