JEE Advance - Chemistry (2019 - Paper 1 Offline - No. 6)
A tin chloride Q undergoes the following reactions (not balanced)
$$Q + C{l^ - } \to X$$
$$Q + M{e_3}N \to Y$$
$$Q + CuC{l_2} \to Z + CuCl$$
X is a monoanion having pyramidal geometry. Both Y and Z are neutral compounds.
Choose the correct option(s).
$$Q + C{l^ - } \to X$$
$$Q + M{e_3}N \to Y$$
$$Q + CuC{l_2} \to Z + CuCl$$
X is a monoanion having pyramidal geometry. Both Y and Z are neutral compounds.
Choose the correct option(s).
There is a coordinate bond in Y
The central atom in Z has one lone pair of electrons.
The oxidation state of the central atom in Z is +2
The central atom in X is sp3 hybridised
Explanation
Sn can exist in +2 or +4 oxidation state. So, Q in the given reactions can be SnCl2 or SnCl4. Since X is monoanion having trigonal pyramidal geometry (sp3 with one lone pair as per VSEPR), so Q is SnCl2, and the first reaction is

The second reaction is Stephen’s reaction, where nitrile is converted to aldehyde via formation of iminium salt.
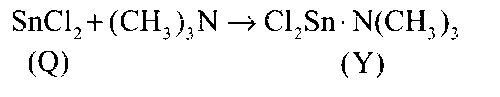

There is a coordinate bond in the Cl2Sn.N(CH3)3 in between nitrogen and Sn metal.
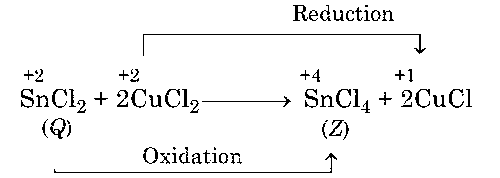
Z is oxidised product and oxidation state of Sn is +4 in Z compound. Structure of SnCl4 (Z) is


The second reaction is Stephen’s reaction, where nitrile is converted to aldehyde via formation of iminium salt.
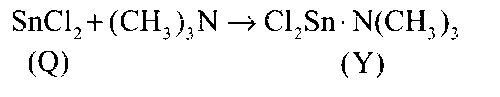

There is a coordinate bond in the Cl2Sn.N(CH3)3 in between nitrogen and Sn metal.

Z is oxidised product and oxidation state of Sn is +4 in Z compound. Structure of SnCl4 (Z) is

Comments (0)


