JEE Advance - Chemistry (2019 - Paper 1 Offline - No. 4)
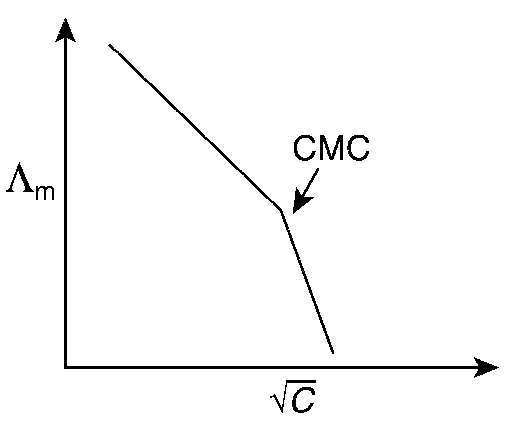
Molar conductivity ($$\Lambda $$m) of aqueous solution of sodium stearate, which behaves as a strong electrolyte, is recorded at varying concentrations (C) of sodium stearate. Which one of the following plots provides the correct representation of micelle formation in the solution?
(critical micelle concentration (CMC) is marked with an arrow in the figures)
(critical micelle concentration (CMC) is marked with an arrow in the figures)



Explanation
At normal or low concentration, sodium stearate (CH3(CH2)16COO-Na+] behaves as strong electrolyte and for strong electrolyte, molar conductance ($$\Lambda $$m) decreases with increase in concentration. Above particular concentration, sodium stearate forms aggregates known as micelles. The concentration is called as CMC. Since, number of ions decreases and hence $$\Lambda $$m also decreases.
Hence, option (c) is correct.
Hence, option (c) is correct.
Comments (0)


