JEE Advance - Chemistry (2019 - Paper 1 Offline - No. 2)
The green colour produced in the borax bead test of a chromium (III) salt is due to
Cr2O3
CrB
Cr(BO2)3
Cr2(B4O7)3
Explanation
Borax bead test is performed only for coloured salt. Borax (sodium pyroborate), Na2B4O7.10H2O on heating gets fused and lose water of crystallisation. It swells up into fluffy white porous mass which melts into a colourless liquid which later form a clear transparent glassy bead consisting of boric anhydride and sodium metaborate.
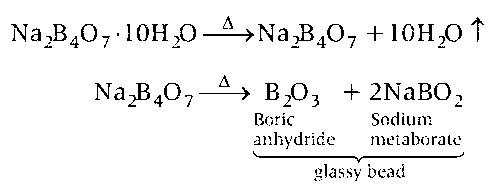
Boric anhydride is non-volatile. When it react with Cr(III) salt then deep green complex is formed.
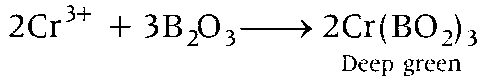
Hence, option (c) is correct.
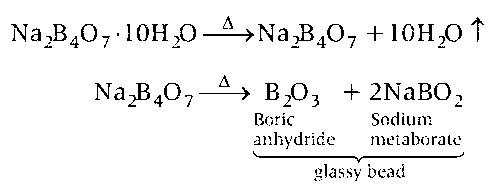
Boric anhydride is non-volatile. When it react with Cr(III) salt then deep green complex is formed.
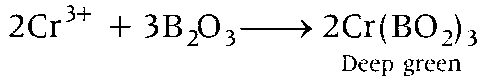
Hence, option (c) is correct.
Comments (0)


