JEE Advance - Chemistry (2019 - Paper 1 Offline - No. 16)
For the following reaction, the equilibrium constant Kc at 298 K is 1.6 $$ \times $$ 1017.
Fe2+(aq) + S2-(aq) ⇌ FeS(s)
When equal volumes of
0.06 M Fe2+(aq) and 0.2 M S2$$ - $$(aq)
solutions are mixed, the equilibrium concentration of Fe2+(aq) is found by Y $$ \times $$ 10$$ - $$17 M. The value of Y is .................
Fe2+(aq) + S2-(aq) ⇌ FeS(s)
When equal volumes of
0.06 M Fe2+(aq) and 0.2 M S2$$ - $$(aq)
solutions are mixed, the equilibrium concentration of Fe2+(aq) is found by Y $$ \times $$ 10$$ - $$17 M. The value of Y is .................
Answer
8.9
Explanation
Given, equilibrium constant (Kc) at 298 K = 1.6 $$ \times $$ 1017
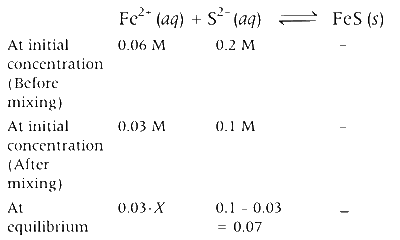
[Here, Kc >>103, thus limiting reagent will be consumed almost completely, 0.03 $$ - $$ X = 0 $$ \therefore $$ X = 0.03]
From equilibrium constant,
$${K_c} = {{[FeS]} \over {[F{e^{2 + }}][{S^{2 - }}]}}$$
$${K_c} = {1 \over {X \times 0.07}}$$
[For $$\mathop {Fes(s)}\limits_{(Pure\,solid)} = 1\,mol\,{L^{ - 1}}]$$]
$$1.6 \times {10^{17}} = {1 \over {X \times 0.07}}$$
$$X = {1 \over {1.6 \times {{10}^{17}} \times 0.07}}$$
$$ = 8.9 \times {10^{ - 17}}$$
Given, X = Y $$ \times $$ 10-17 = 8.9 $$ \times $$ 10-17
$$ \therefore $$ Y = 8.9

[Here, Kc >>103, thus limiting reagent will be consumed almost completely, 0.03 $$ - $$ X = 0 $$ \therefore $$ X = 0.03]
From equilibrium constant,
$${K_c} = {{[FeS]} \over {[F{e^{2 + }}][{S^{2 - }}]}}$$
$${K_c} = {1 \over {X \times 0.07}}$$
[For $$\mathop {Fes(s)}\limits_{(Pure\,solid)} = 1\,mol\,{L^{ - 1}}]$$]
$$1.6 \times {10^{17}} = {1 \over {X \times 0.07}}$$
$$X = {1 \over {1.6 \times {{10}^{17}} \times 0.07}}$$
$$ = 8.9 \times {10^{ - 17}}$$
Given, X = Y $$ \times $$ 10-17 = 8.9 $$ \times $$ 10-17
$$ \therefore $$ Y = 8.9
Comments (0)


