JEE Advance - Chemistry (2019 - Paper 1 Offline - No. 12)
Each of the following options contains a set of four molecules. Identify the option(s) where all four molecules posses permanent dipole moment at room temperature.
SO2, C6H5Cl, H2Se, BrF5
BeCl2, CO2, BCl3, CHCl3
NO2, NH3, POCl3, CH3Cl
BF3, O3, SF6, XeF6
Explanation
The molecules which gives permanent dipole moment are polar in nature.
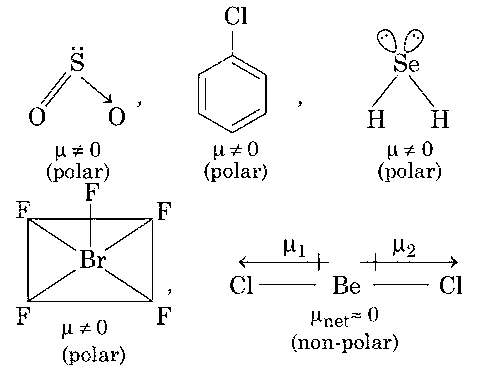
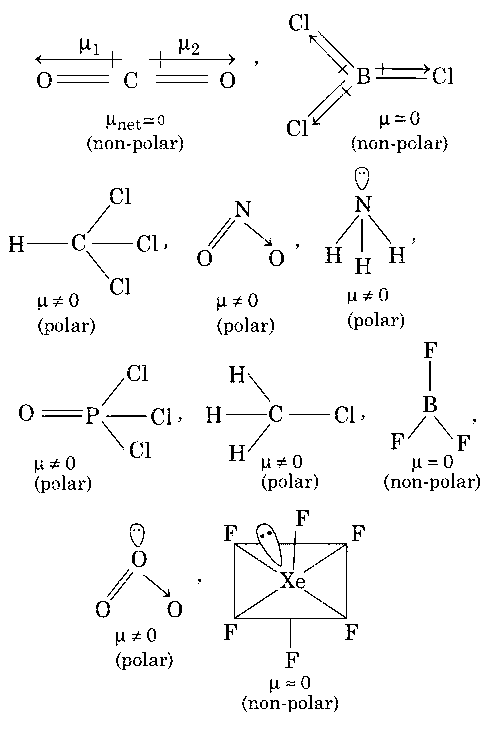
Thus, options (a, c) are correct.


Thus, options (a, c) are correct.
Comments (0)


