JEE Advance - Chemistry (2019 - Paper 1 Offline - No. 11)
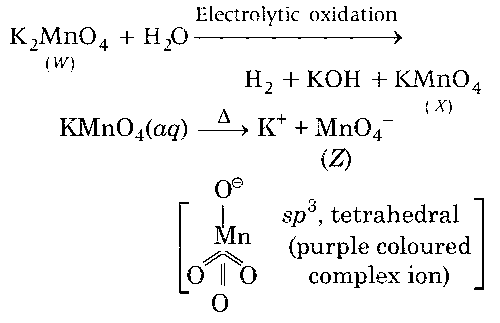
Fusion of MnO2 with KOH in presence of O2 produces a salt W. Alkaline solution of W upon electrolytic oxidation yields another salt X. The manganese containing ions present in W and X, respectively, are Y and Z. Correct statement(s) is (are)
Both Y and Z are coloured and have tetrahedral shape
Y is diamagnetic in nature while Z is paramagnetic
In both Y and Z, $$\pi $$-bonding occurs between p-orbitals of oxygen and d-orbitals of manganese
In aqueous acidic solution, Y undergoes disproportionation reaction to give Z and MnO2
Explanation
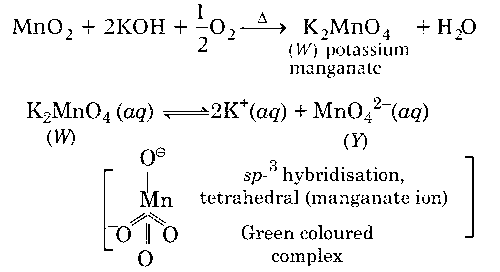
$$MnO_4^{2 - }$$ ion has one unpaired electrons, therefore it gives d-d transition to form green colour. Y complex has paramagnetic nature due to presence of one unpaired electron.
In aqueous solution,
$$MnO_4^{2 - }$$ ions gives charge transfer spectrum in which a fraction of electronic charge is transferred between the molecular entities.
$$ \because $$ $$\mathop {MnO_4^{2 - }}\limits_{(Y)} \mathrel{\mathop{\kern0pt\longrightarrow} \limits_{oxidation}^{Electrolytic}} \mathop {MnO_4^ - }\limits_{(Z)} + {e^ - }$$
In acidic medium, Y undergoes disproportionation reaction.
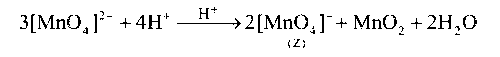
$$\mathop {MnO_4^{2 - }}\limits_{(Y)} $$ and $$\mathop {MnO_4^ - }\limits_{(Z)} $$ both ions form $$\pi $$-bonding between p-orbitals of oxygen and d-orbitals of manganese.
Thus, options (a, c, d) are correct.
Comments (0)


