JEE Advance - Chemistry (2019 - Paper 1 Offline - No. 10)
Which of the following statement(s) is(are) true?
The two six-membered cyclic hemiacetal forms of D-(+)- glucose are called anomers.
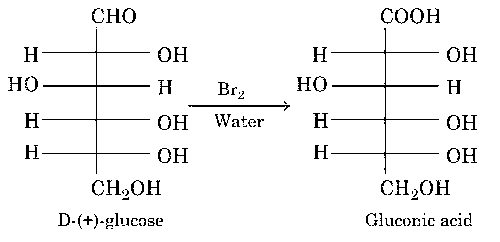
Oxidation of glucose with bromine water gives glutamic acid
Monosaccharides cannot be hydrolysed to given polyhydroxy aldehydes and ketones
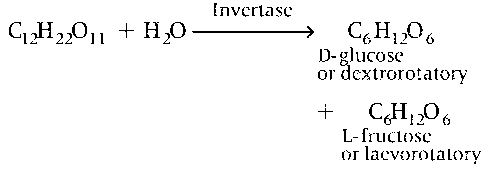
Hydrolysis of sucrose gives dextrorotatory glucose and laevorotatory fructose
Explanation
The explanation of given statements are as follows :
(a) Two six membered cyclic hemiacetal form of D-(+)- glucose are called anomers.
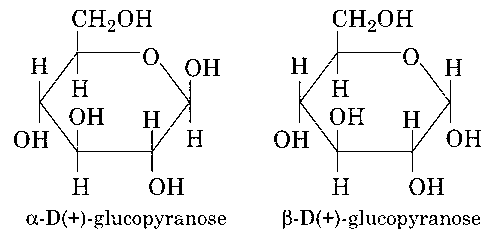
Both are anomers.
(b) Oxidation of glucose in presence of Br2 water gives gluconic acid.
(c) Monosaccharides can not be hydrolysed into polyhydroxy aldehydes and ketones.
(d) Hydrolysis of sucrose gives D-glucose and L-fructose.
Hence, options (a, c, d) are correct.
(a) Two six membered cyclic hemiacetal form of D-(+)- glucose are called anomers.

Both are anomers.
(b) Oxidation of glucose in presence of Br2 water gives gluconic acid.

(c) Monosaccharides can not be hydrolysed into polyhydroxy aldehydes and ketones.
(d) Hydrolysis of sucrose gives D-glucose and L-fructose.

Hence, options (a, c, d) are correct.
Comments (0)


