JEE Advance - Chemistry (2019 - Paper 1 Offline - No. 1)
The correct order of acid strength of the following carboxylic acids is
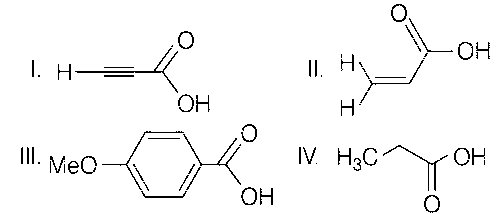

III > II > I > IV
I > II > III > IV
II > I > IV > III
I > III > II > IV
Explanation
Acidic nature depends upon nature of electron withdrawing group and electronegativity. Electronegativity further depends on % of s character. Higher the s-character, greater will be the electronegativity and hence tendency to loose H increases thus acidic character also increases.
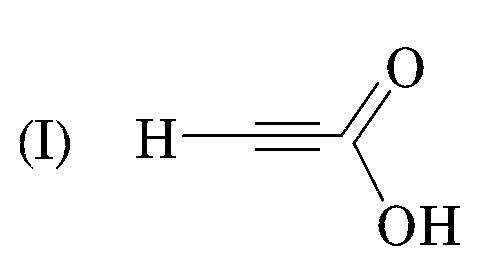
sp- hybridisation (50% s character) (pKa = 1.86)
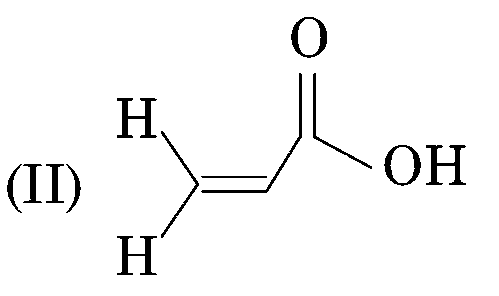
sp2 - hybridisation (33-33% s character) (pKa = 4.3)
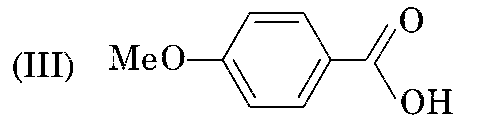
sp2 - hybridisation (Resonance effect) (pKa = 4.5)
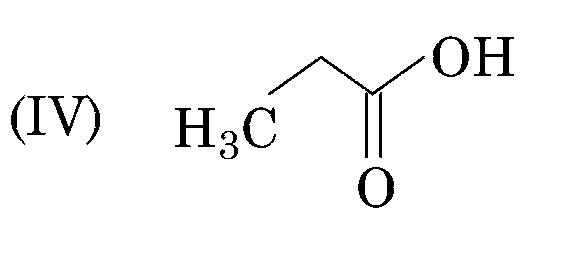
sp3 - hybridisation (25% s-character) (pKa = 4.8)
Hence, acidic order I > II > III > IV.
II is more acidic than III since electron donating group ($$ - OC{H_3}$$) is attached to benzene ring in III which decreases the acidic character.
On the other hand, pKa value also determined acidic nature, lower pKa value gives maximum acidic character.
Hence, option (b) is correct.

sp- hybridisation (50% s character) (pKa = 1.86)

sp2 - hybridisation (33-33% s character) (pKa = 4.3)

sp2 - hybridisation (Resonance effect) (pKa = 4.5)

sp3 - hybridisation (25% s-character) (pKa = 4.8)
Hence, acidic order I > II > III > IV.
II is more acidic than III since electron donating group ($$ - OC{H_3}$$) is attached to benzene ring in III which decreases the acidic character.
On the other hand, pKa value also determined acidic nature, lower pKa value gives maximum acidic character.
Hence, option (b) is correct.
Comments (0)


