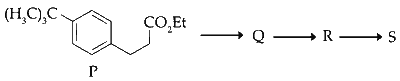JEE Advance - Chemistry (2017 - Paper 2 Offline - No. 7)
The reactions, $$Q$$ to $$R$$ and $$R$$ to $$S,$$ are
Dehydration and Friedel$$-$$Crafts acylation
Aromatic sulfonation and Friedel$$-$$Crafts acylation
Friedel$$-$$Crafts alkylation, dehydration and Friedel$$-$$Crafts acylation
Friedel$$-$$Crafts alkylation and Friedel$$-$$Crafts acylation
Explanation
The reaction $\mathrm{Q} \rightarrow \mathrm{R}$ and $\mathrm{R} \rightarrow \mathrm{S}$ Friedel-Crafts alkylation and Friedel-Crafts alkylation is the introduction of alkyl group into benzene ring in presence of lewis acid catalyst such as anhydrous $\mathrm{AlCl}_3$. Friedel Crafts acylation is the introduction of acyl group into benzene ring in presence of lewis acid catalyst such as anhydrous $\mathrm{AlCl}_3$.
Comments (0)