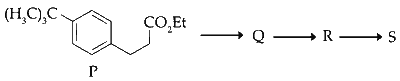JEE Advance - Chemistry (2017 - Paper 2 Offline - No. 3)
The product $$S$$ is
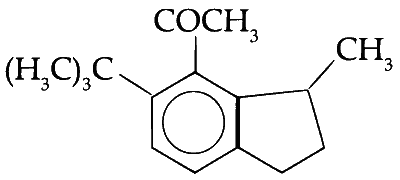
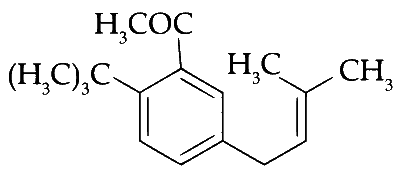
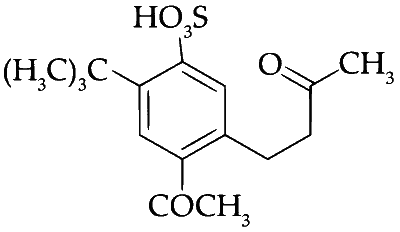
Explanation
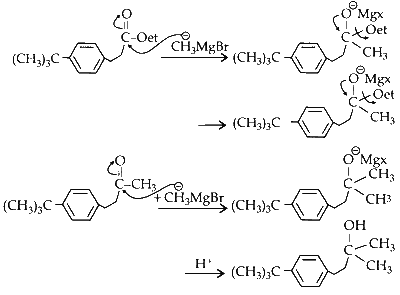
(i) Compound P reacts with $\mathrm{CH}_3 \mathrm{MgBr}$ in excess in $\left(\mathrm{C}_2 \mathrm{H}_5\right)_2 \mathrm{O}$ followed by addition of water to form alcohol derivative of the compound.
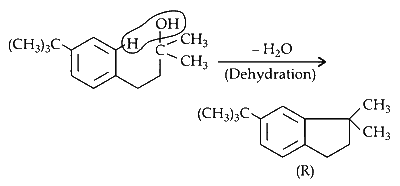
(ii) Compound $Q$ undergoes dehydration in presence of $\mathrm{H}_2 \mathrm{SO}_4$ at $0^{\circ} \mathrm{C}$.
(iii) Compound $R$ undergoes acylation of aromatic ring in presence of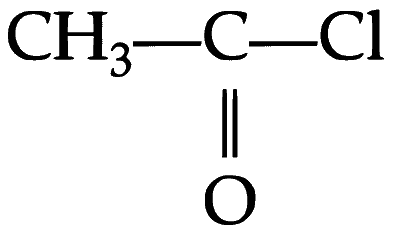 and anhydrous $\mathrm{AlCl}_3$.
and anhydrous $\mathrm{AlCl}_3$.
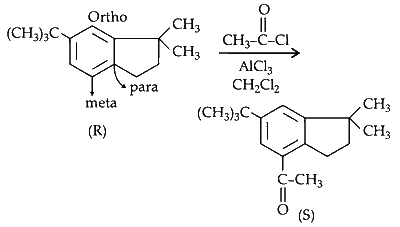
The substitution should take place at ortho or para position but due to steric crowding at ortho and occupancy of para position. Meta position is substituted with acetyl group.

(ii) Compound $Q$ undergoes dehydration in presence of $\mathrm{H}_2 \mathrm{SO}_4$ at $0^{\circ} \mathrm{C}$.

(iii) Compound $R$ undergoes acylation of aromatic ring in presence of
 and anhydrous $\mathrm{AlCl}_3$.
and anhydrous $\mathrm{AlCl}_3$.
The substitution should take place at ortho or para position but due to steric crowding at ortho and occupancy of para position. Meta position is substituted with acetyl group.
Comments (0)