JEE Advance - Chemistry (2017 - Paper 2 Offline - No. 10)
Among the following, the correct statement(s) is (are)
$$Al{\left( {C{H_3}} \right)_3}$$ has the three-centre two-electron bonds in its dimeric structure
$$B{H_3}$$ has the three-center two-electron bonds in its dimeric structure
$$AlC{l_3}$$ has the three-center two-electron bonds in its dimeric structure
The Lewis acidity of $$BC{l_3}$$ is greater than that of $$AlC{l_3}$$
Explanation
Option (A): Correct.
The aluminium compounds are unusual because they have dimeric structures, and appear to have three-centre bonds involving $s p^3$ hybrid orbitals on $\mathrm{Al}$ and $\mathrm{C}$ in $\mathrm{Al}-\mathrm{C}-\mathrm{Al}$ bridges.
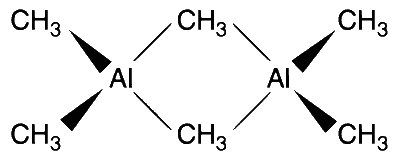
Option (B): Correct.
In diborane $\left(\mathrm{BH}_3\right)$ there are 12 valency electrons, three from each $B$ atom and six from the $\mathrm{H}$ atoms. An $s p^3$ hybrid orbital from each boron atom overlaps with the $1 s$ orbital of the hydrogen. This gives a delocalised molecular orbital covering all three nuclei, containing one pair of electrons and making up one of the bridges. This is a three-centre two-electron bond $(3 c-2 e)$.
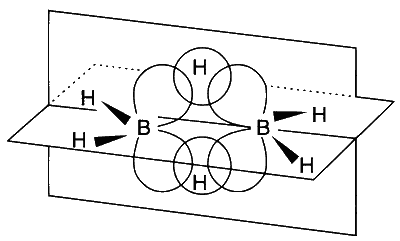
Option (D): Group 13 elements have only three valency electrons. When these are used to form three covalent bonds, the atom has a share in only six electrons. The compounds are therefore electron deficient. In $\mathrm{AlCl}_3$, effective $\pi$ overlap takes place between $p$ orbitals of $\mathrm{Al}$ and $\mathrm{Cl}$ due to their comparable size while in $\mathrm{BCl}_3$, the $\pi$ overlap is not effective as $p$ orbital of boron is smaller than that of $p$ orbital of chlorine, hence, the acidity of $\mathrm{BCl}_3$ is greater than that of $\mathrm{AlCl}_3$.
The aluminium compounds are unusual because they have dimeric structures, and appear to have three-centre bonds involving $s p^3$ hybrid orbitals on $\mathrm{Al}$ and $\mathrm{C}$ in $\mathrm{Al}-\mathrm{C}-\mathrm{Al}$ bridges.

Option (B): Correct.
In diborane $\left(\mathrm{BH}_3\right)$ there are 12 valency electrons, three from each $B$ atom and six from the $\mathrm{H}$ atoms. An $s p^3$ hybrid orbital from each boron atom overlaps with the $1 s$ orbital of the hydrogen. This gives a delocalised molecular orbital covering all three nuclei, containing one pair of electrons and making up one of the bridges. This is a three-centre two-electron bond $(3 c-2 e)$.

Option (D): Group 13 elements have only three valency electrons. When these are used to form three covalent bonds, the atom has a share in only six electrons. The compounds are therefore electron deficient. In $\mathrm{AlCl}_3$, effective $\pi$ overlap takes place between $p$ orbitals of $\mathrm{Al}$ and $\mathrm{Cl}$ due to their comparable size while in $\mathrm{BCl}_3$, the $\pi$ overlap is not effective as $p$ orbital of boron is smaller than that of $p$ orbital of chlorine, hence, the acidity of $\mathrm{BCl}_3$ is greater than that of $\mathrm{AlCl}_3$.
Comments (0)


