JEE Advance - Chemistry (2017 - Paper 2 Offline - No. 1)
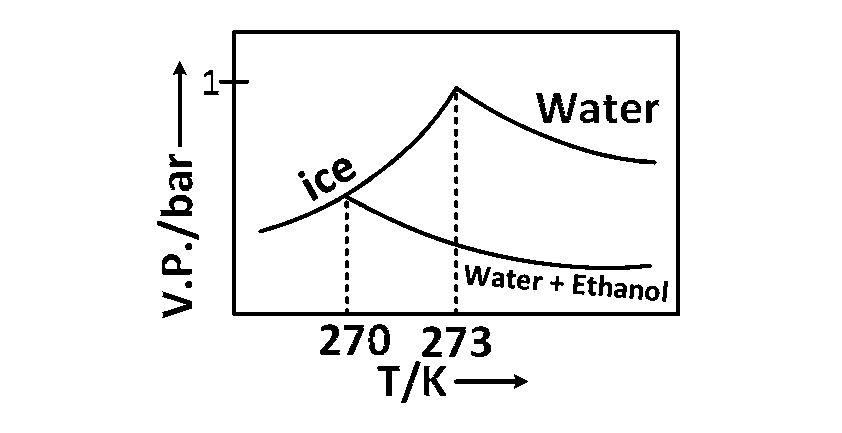
Pure water freezes at $$273$$ $$K$$ and $$1$$ bar. The addition of $$34.5$$ $$g$$ of ethanol to $$500$$ $$g$$ of water changes the freezing point of the solution. Use the freezing point depression constant of water as $$2$$ kg $$mo{l^{ - 1}}.$$ The figures shown below represent plots of vapor pressure $$(V.P.)$$ versus temperature $$(T).$$ [molecular weight of ethanol is $$46$$ $$g$$ $$mo{l^{ - 1}}.$$ ] Among the following, the option representing change in the freezing point is

Explanation
Depression in freezing point is given by $$\Delta$$Tf = Kfm
where $$m = {{1000 \times {w_{solute}}} \over {{M_{solute}} \times {w_{solvent}}}}$$
Substituting the values, we get
$$\Delta {T_f} = 2 \times {{1000 \times 34.5} \over {46 \times 500}} = 3K$$
Therefore, Tf = 270 K and $$T_f^o$$ = 273 K. Hence, option (c) indicates correct representation of the graph.
Comments (0)


