JEE Advance - Chemistry (2017 - Paper 1 Offline - No. 8)
The sum of the number of lone pairs of electrons on each central atom in the following species is
$${[TeB{r_6}]^{2 - }},{\left[ {Br{F_2}} \right]^ + },SNF_3,$$ and $${\left[ {Xe{F_3}} \right]^ - }$$
(Atomic numbers: $$N = 7,F = 9,$$ $$S = 16,Br = 35,$$ $$Te = 52,Xe = 54$$)
$${[TeB{r_6}]^{2 - }},{\left[ {Br{F_2}} \right]^ + },SNF_3,$$ and $${\left[ {Xe{F_3}} \right]^ - }$$
(Atomic numbers: $$N = 7,F = 9,$$ $$S = 16,Br = 35,$$ $$Te = 52,Xe = 54$$)
Answer
6
Explanation
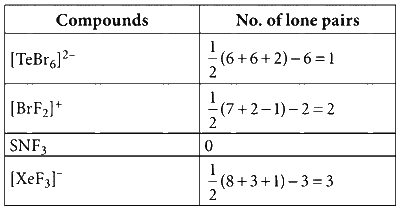
Sum of number of lone pairs = 1 + 2 + 0 + 3 = 6
Comments (0)


