JEE Advance - Chemistry (2017 - Paper 1 Offline - No. 5)
Addition of excess aqueous ammonia to a pink colored aqueous solution of $$MC{l_2},6{H_2}O\left( X \right)$$ and $$N{H_4}Cl$$ gives an octahedral complex $$Y$$ in the presence of air. In aqueous solution, complex $$Y$$ behaves as $$1:3$$ electrolyte. The reaction of $$X$$ with excess $$HCl$$ at room temperature results in the formation of a blue colored complex $$Z.$$ The calculated spin only magnetic moment of $$X$$ and $$Z$$ is $$3.87$$ $$B.M.,$$ whereas it is zero for complex $$Y.$$ Among the following options, which statement(s) is (are) correct?
Addition of silver nitrate to $$Y$$ gives only two equivalents of silver chloride
The hybridization of the central metal ion in $$Y$$ is $${d^2}s{p^3}$$
$$Z$$ is a tetrahedral complex
When $$X$$ and $$Z$$ are in equilibrium at $${0^ \circ }C,$$ the color of the solution is pink
Explanation
The pink coloured aqueous solution of $\mathrm{MCl}_2 \cdot 6 \mathrm{H}_2 \mathrm{O}$ has central metal atom bonded six water molecule by co-ordinate bonds and there are two primary valences of chloride ions. The formula and structure of the complex $X$ can be represented as :
Formula : $\left[\mathrm{M}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right] \mathrm{Cl}_2$
$$ \left[\mathrm{M}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right] \mathrm{Cl}_2 \rightarrow\left[\mathrm{M}\left(\mathrm{H}_2 \mathrm{O}\right)\right]^{2+}+2 \mathrm{Cl}^{-} $$
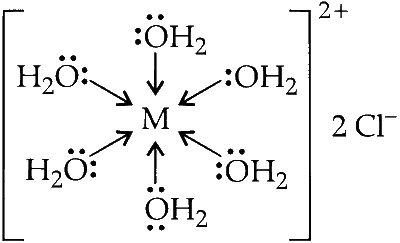
Here, the six non ionisable water molecules are the secondary valence and two ionisable chloride ions are primary valence. Since, the hexa coordinated aqua complex of cobalt is pink in colour; hence, central metal is cobalt.
(i) Reaction of the complex X with ammonium chloride in presence of air gives hexamminecobalt (III) chloride which is a 1: 3 electrolyte.

$$ \begin{aligned} & \text { Option (A) : } \\\\ & {\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right] \mathrm{Cl}_3+3 \mathrm{AgNO}_3 \rightarrow\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]\left(\mathrm{NO}_3\right)_3+3 \mathrm{AgCl}} \end{aligned} $$
Option (B) : $\mathrm{NH}_3$ is a strong field ligand, therefore, pairing of electrons will take place.

Option (C) :

Option (D) : When X and Z are in equilibrium at 0°C
$$ \underset{\text{Pink}}{\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]}\rightarrow \underset{\text{Blue}}{\left[\mathrm{CoCl}_4\right]^{2-}} $$
Since, formation of chlorine complex takes place in presence of excess chlorine at room temperature $\left(25^{\circ} \mathrm{C}\right)$. At lower temperature $\left(0^{\circ} \mathrm{C}\right)$, the equilibrium is more shifted towards left favouring the formation of pink coloured hexaaquacobalt (II) chloride complex.
Option (D) is correct.
Formula : $\left[\mathrm{M}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right] \mathrm{Cl}_2$
$$ \left[\mathrm{M}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right] \mathrm{Cl}_2 \rightarrow\left[\mathrm{M}\left(\mathrm{H}_2 \mathrm{O}\right)\right]^{2+}+2 \mathrm{Cl}^{-} $$

Here, the six non ionisable water molecules are the secondary valence and two ionisable chloride ions are primary valence. Since, the hexa coordinated aqua complex of cobalt is pink in colour; hence, central metal is cobalt.
(i) Reaction of the complex X with ammonium chloride in presence of air gives hexamminecobalt (III) chloride which is a 1: 3 electrolyte.

$$ \begin{aligned} & \text { Option (A) : } \\\\ & {\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right] \mathrm{Cl}_3+3 \mathrm{AgNO}_3 \rightarrow\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]\left(\mathrm{NO}_3\right)_3+3 \mathrm{AgCl}} \end{aligned} $$
Option (B) : $\mathrm{NH}_3$ is a strong field ligand, therefore, pairing of electrons will take place.

Option (C) :

Option (D) : When X and Z are in equilibrium at 0°C
$$ \underset{\text{Pink}}{\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]}\rightarrow \underset{\text{Blue}}{\left[\mathrm{CoCl}_4\right]^{2-}} $$
Since, formation of chlorine complex takes place in presence of excess chlorine at room temperature $\left(25^{\circ} \mathrm{C}\right)$. At lower temperature $\left(0^{\circ} \mathrm{C}\right)$, the equilibrium is more shifted towards left favouring the formation of pink coloured hexaaquacobalt (II) chloride complex.
Option (D) is correct.
Comments (0)


