JEE Advance - Chemistry (2017 - Paper 1 Offline - No. 3)
The correct statement(s) about the oxoacids, $$HCl{O_4}$$ and $$HClO$$ is (are)
The central atom in both $$HCl{O_4}$$ and $$HClO$$ is $$s{p^3}$$ hybridized
$$HCl{O_4}$$ is more acidic than $$HClO$$ because of the resonance stabilization of its anion
$$HCl{O_4}$$ is formed in the reaction between $$C{l_2}$$ and $${H_2}O$$
The conjugate base of $$HCl{O_4}$$ is weaker base than $${H_2}O$$
Explanation
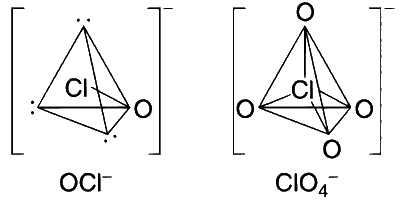
Option (A): Correct. The structures of the ions formed are shown in below figure. All these structures are based on a tetrahedron. The $s p^3$ hybrid orbitals used for bonding form only weak $\sigma$ bonds, because the $s$ and $p$ levels differ appreciably in energy. The ions are stabilised by strong $p \pi-d \pi$ bonding between full $2 p$ orbitals on oxygen with empty $d$ orbitals on the halogen atoms.
Option (B): $\mathrm{HClO}_4$ is an extremely strong acid, while $\mathrm{HOCl}$ is a very weak acid. Oxygen is more electronegative than chlorine. The more oxygen atoms that are bonded, the more the electrons will be pulled away from the $\mathrm{O}-\mathrm{H}$ bond, and the more this bond will be weakened. Thus $\mathrm{HClO}_4$ requires the least energy to break the $\mathrm{O}-\mathrm{H}$ bond and form $\mathrm{H}^{+}$.
Option (C): The reaction is $\mathrm{Cl}_2+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{HOCl}+\mathrm{HCl}$
Option (D): $\mathrm{As} ~\mathrm{HClO}_4$ is a stronger acid than $\mathrm{H}_2 \mathrm{O}$, therefore, its conjugate base $\left(\mathrm{ClO}_4^{-}\right)$will be weaker than $\left(\mathrm{OH}^{-}\right)$that of $\mathrm{H}_2 \mathrm{O}$.
$$ \mathrm{HClO}_4+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{H}_3 \mathrm{O}^{+}+\mathrm{ClO}_4^{-} $$

Option (B): $\mathrm{HClO}_4$ is an extremely strong acid, while $\mathrm{HOCl}$ is a very weak acid. Oxygen is more electronegative than chlorine. The more oxygen atoms that are bonded, the more the electrons will be pulled away from the $\mathrm{O}-\mathrm{H}$ bond, and the more this bond will be weakened. Thus $\mathrm{HClO}_4$ requires the least energy to break the $\mathrm{O}-\mathrm{H}$ bond and form $\mathrm{H}^{+}$.
Option (C): The reaction is $\mathrm{Cl}_2+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{HOCl}+\mathrm{HCl}$
Option (D): $\mathrm{As} ~\mathrm{HClO}_4$ is a stronger acid than $\mathrm{H}_2 \mathrm{O}$, therefore, its conjugate base $\left(\mathrm{ClO}_4^{-}\right)$will be weaker than $\left(\mathrm{OH}^{-}\right)$that of $\mathrm{H}_2 \mathrm{O}$.
$$ \mathrm{HClO}_4+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{H}_3 \mathrm{O}^{+}+\mathrm{ClO}_4^{-} $$
Comments (0)


