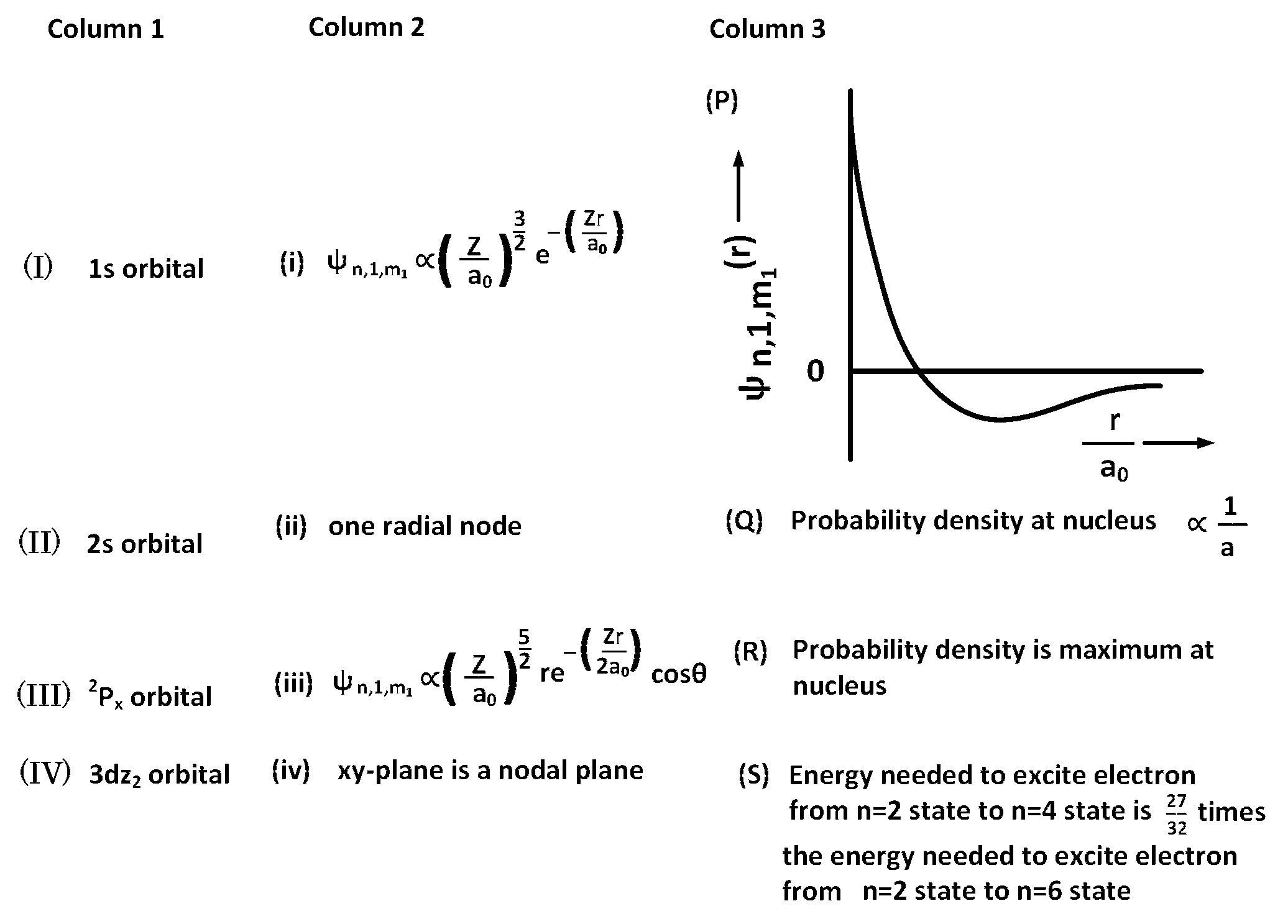
JEE Advance - Chemistry (2017 - Paper 1 Offline - No. 18)
For the given orbital in Column 1, the only CORRECT combination for any hydrogen-like species is :
(I) (ii) (S)
(IV) (iv) (R)
(II) (ii) (P)
(III) (iii) (P)
Explanation
- Option (A) : Incorrect. The total number of radial nodes is given by $n-l-1$. For $1 s$ orbital, the radial node is zero (i.e. $1-0-1=0$ ).
- Option (B) : Incorrect. $d_{z^2}$ orbital has two lobes that point in opposite directions along $z$-axis plus a doughnut-shaped ring of electron density around the centre that lies in the $x y$-plane. Therefore, it does not have any nodal plane.
- Option (C) : Correct. The total number of radial nodes is given by $n-l-1$.
For $2 s$ : Radial nodes $=2-0-1=1$
The orbital 2s of hydrogen like species can be diagrammatically represented as :
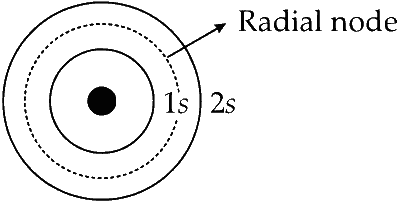
There is a spherical region of zero electron density between $1 s$ and the $2 s$ orbital of hydrogen like species. This spherical region is called the node. Only one radial node exist between $1 s$ and $2 s$.
The plot of wave function for $2 s$ verses radial distance from the nucleus is represented as
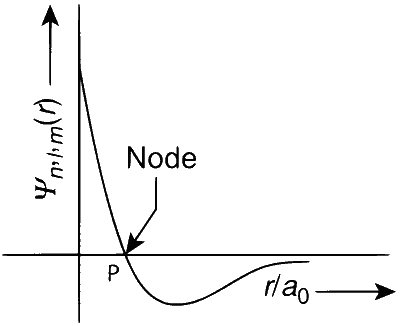
Point $P$ represents the region of zero electron density at a distance $r$ from the nucleus. This represents spherical node.
- Option (D) : Incorrect. The probability density at nucleus is for $2p$ orbital is zero.
- Option (B) : Incorrect. $d_{z^2}$ orbital has two lobes that point in opposite directions along $z$-axis plus a doughnut-shaped ring of electron density around the centre that lies in the $x y$-plane. Therefore, it does not have any nodal plane.
- Option (C) : Correct. The total number of radial nodes is given by $n-l-1$.
For $2 s$ : Radial nodes $=2-0-1=1$
The orbital 2s of hydrogen like species can be diagrammatically represented as :

There is a spherical region of zero electron density between $1 s$ and the $2 s$ orbital of hydrogen like species. This spherical region is called the node. Only one radial node exist between $1 s$ and $2 s$.
The plot of wave function for $2 s$ verses radial distance from the nucleus is represented as

Point $P$ represents the region of zero electron density at a distance $r$ from the nucleus. This represents spherical node.
- Option (D) : Incorrect. The probability density at nucleus is for $2p$ orbital is zero.
Comments (0)