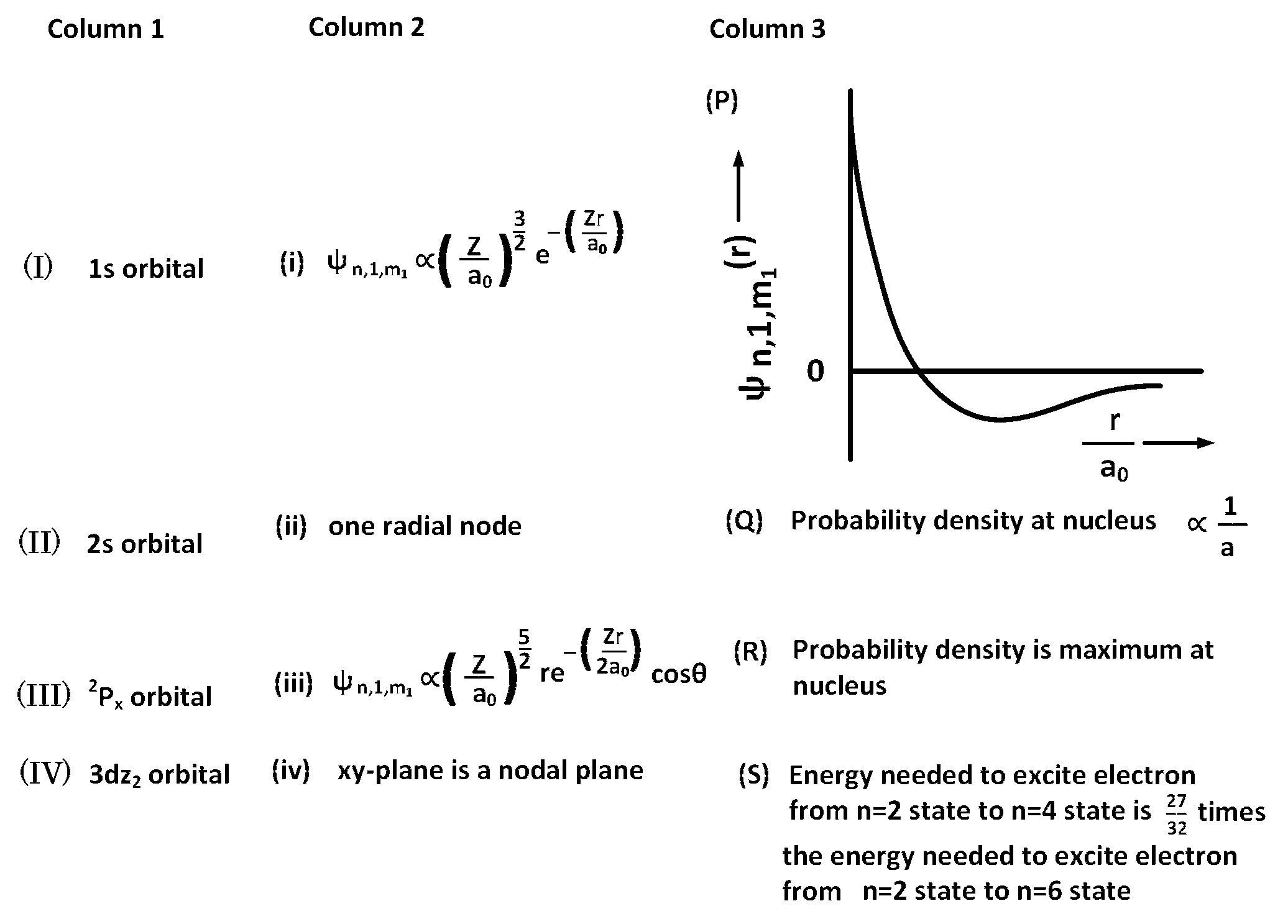
JEE Advance - Chemistry (2017 - Paper 1 Offline - No. 16)
For $$H{e^ + }$$ ion, the only INCORRECT combination is
(I) (i) (R)
(II) (ii) (Q)
(I) (iii) (R)
(I) (i) (S)
Explanation
The ion $\mathrm{He}^{+}$having 1 electron (in $1 s$ orbital) is hydrogen like species; hence, its $1 s$ orbital will have:
(i) Wave function same as that of hydrogen, i.e.,
$$ \psi_{1,0,0}=\left(\frac{Z}{a_0}\right)^{3 / 2} e^{-Z r / a_0} $$
(ii) The probability density for the electron in the $1 s$ orbital is represented as:

For $1 s$ orbital probability density is maximum at the nucleus.
(iii) Probability density for $2 s$ orbitals is given by
$$ =\frac{1}{2 a_0^{3 / 2}}\left(1-\frac{r}{2 a_0}\right) e^{-r / 2 a_0} $$
Probability density $\propto \frac{1}{a_0^3}$
(iv) For electron in the $1 s$ orbital, the wave function of electron is not the function of angular wave function i.e., $\theta$ and $\phi$. Hence, 1 s orbital cannot have the wave function
$$ \psi_{n, l, m_l} \propto\left(\frac{Z}{a_0}\right)^{5 / 2} r e^{\left(-Z r / 2 a_0\right) \cos \theta} $$
(i) Wave function same as that of hydrogen, i.e.,
$$ \psi_{1,0,0}=\left(\frac{Z}{a_0}\right)^{3 / 2} e^{-Z r / a_0} $$
(ii) The probability density for the electron in the $1 s$ orbital is represented as:

For $1 s$ orbital probability density is maximum at the nucleus.
(iii) Probability density for $2 s$ orbitals is given by
$$ =\frac{1}{2 a_0^{3 / 2}}\left(1-\frac{r}{2 a_0}\right) e^{-r / 2 a_0} $$
Probability density $\propto \frac{1}{a_0^3}$
(iv) For electron in the $1 s$ orbital, the wave function of electron is not the function of angular wave function i.e., $\theta$ and $\phi$. Hence, 1 s orbital cannot have the wave function
$$ \psi_{n, l, m_l} \propto\left(\frac{Z}{a_0}\right)^{5 / 2} r e^{\left(-Z r / 2 a_0\right) \cos \theta} $$
Comments (0)