JEE Advance - Chemistry (2017 - Paper 1 Offline - No. 1)
The color of the $${X_2}$$ molecules of group $$17$$ elements changes gradually from yellow to violet down the group. This is due to
The physical state of $${X_2}$$ at room temperature changes from gas to solid down the group
Decrease in ionization energy down the group
Decrease in $${\pi ^ * } - {\sigma ^ * }$$ gap down the group
Decrease in HOMO-LUMO gap down the group
Explanation
Halogens exist as diatomic molecule of different colours:
$$ \begin{array}{|c|c|c|} \hline \text { S.No. } & \text { Halogen } & \text { Colour } \\ \hline 1 . & \text { Fluorine } & \text { Pale yellow } \\ \hline 2 . & \text { Chlorine } & \text { Yellow green } \\ \hline 3 . & \text { Bromine } & \text { Red brown } \\ \hline 4 . & \text { Iodine } & \text { Purple } \\ \hline \end{array} $$
(i) The molecular orbital energy level diagram explains the appearance of colour by halogens. The MOT for halogens is represented.
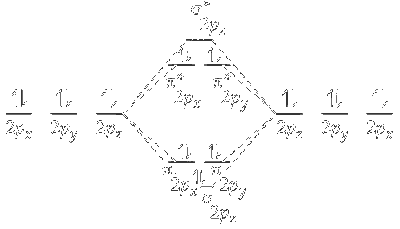
(ii) It represents molecular orbital energy level diagram for fluorine. Similar molecular energy level diagram exist. Other halogens.
(iii) Antibonding $\pi$ orbitals, i.e., $\pi^*{ }_{2 p x}$ and $\pi^*{ }_{2 p y}$ forms the highest occupied molecular orbital (HOMO) and antibonding sigma* orbitals forms the lowest unoccupied molecular orbital (LUMO) for halogens.
(iv) Absorption of energy of suitable wavelength (or colour) results in transition of electron from HOMO to LUMO. As electron returns back to ground state, i.e, HOMO, it releases energy corresponding to a different wavelength (colour complementary to the colour absorbed). This gives halogens their characteristic colour.
(v) As we move down the group 17, size of halogen atom increases and nuclear force of attraction for the outermost shell electrons decrease. This affects the energy gap between $\mathrm{HOMO}$ and LUMO.
$$ \mathrm{F}_2>\mathrm{Cl}_2>\mathrm{Br}_2>\mathrm{I}_2 $$
HOMO-LUMO energy gap decreases $\rightarrow$
Wavelength of light emitted decreases $\rightarrow$
(vi) The energy gap between HOMO and LUMO keeps on decreasing as we move down the group. As a result, the energy required for transition of electron from HOMO to LUMO decreases and less energy (or light of lower wavelength is absorbed).
(vii)When electron moves back to HOMO, energy is emitted. It corresponds to the light of complementary colour to the colour of the light absorbed.
$$ \begin{array}{|c|c|c|} \hline \text { S.No. } & \text { Halogen } & \text { Colour } \\ \hline 1 . & \text { Fluorine } & \text { Pale yellow } \\ \hline 2 . & \text { Chlorine } & \text { Yellow green } \\ \hline 3 . & \text { Bromine } & \text { Red brown } \\ \hline 4 . & \text { Iodine } & \text { Purple } \\ \hline \end{array} $$
(i) The molecular orbital energy level diagram explains the appearance of colour by halogens. The MOT for halogens is represented.


(ii) It represents molecular orbital energy level diagram for fluorine. Similar molecular energy level diagram exist. Other halogens.
(iii) Antibonding $\pi$ orbitals, i.e., $\pi^*{ }_{2 p x}$ and $\pi^*{ }_{2 p y}$ forms the highest occupied molecular orbital (HOMO) and antibonding sigma* orbitals forms the lowest unoccupied molecular orbital (LUMO) for halogens.
(iv) Absorption of energy of suitable wavelength (or colour) results in transition of electron from HOMO to LUMO. As electron returns back to ground state, i.e, HOMO, it releases energy corresponding to a different wavelength (colour complementary to the colour absorbed). This gives halogens their characteristic colour.
(v) As we move down the group 17, size of halogen atom increases and nuclear force of attraction for the outermost shell electrons decrease. This affects the energy gap between $\mathrm{HOMO}$ and LUMO.
$$ \mathrm{F}_2>\mathrm{Cl}_2>\mathrm{Br}_2>\mathrm{I}_2 $$
HOMO-LUMO energy gap decreases $\rightarrow$
Wavelength of light emitted decreases $\rightarrow$
(vi) The energy gap between HOMO and LUMO keeps on decreasing as we move down the group. As a result, the energy required for transition of electron from HOMO to LUMO decreases and less energy (or light of lower wavelength is absorbed).
(vii)When electron moves back to HOMO, energy is emitted. It corresponds to the light of complementary colour to the colour of the light absorbed.
Comments (0)


