JEE Advance - Chemistry (2016 - Paper 1 Offline - No. 6)
The compound(s) with TWO lone pairs of electrons on the central atom is(are)
BrF5
ClF3
XeF4
SF4
Explanation
Structure of each of the compound is
represented as :
(A) BrF5
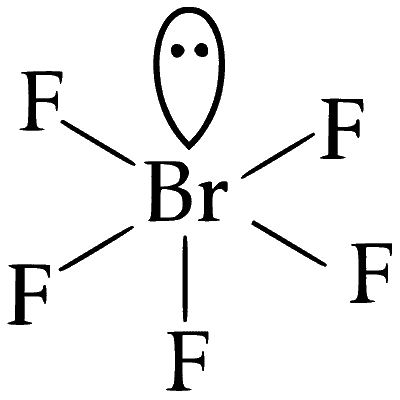
Structure : Square pyramidal
(i) One lone pair
(ii) Four bond pair
(B) ClF3
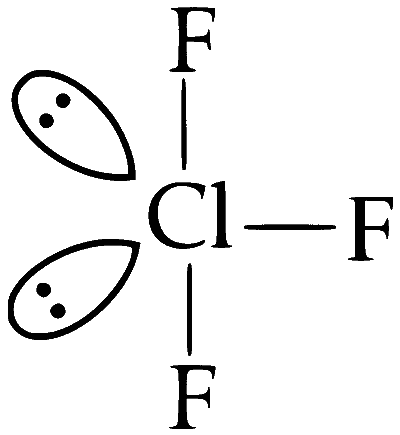
Structure : T-Shaped
(i) Two lone pair
(ii) Three bond pair
(C) XeF4
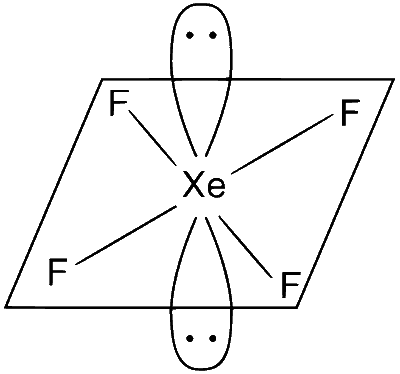
Structure : Square planar
(i) Two lone pair
(ii) Four bond pair
(D) SF4
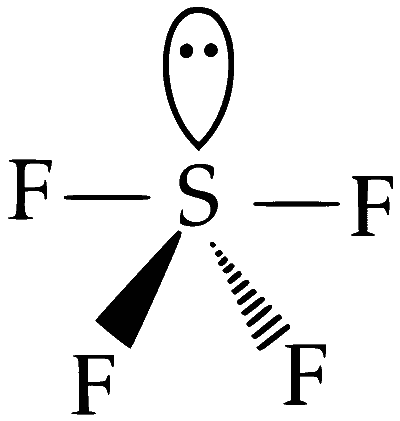
Structure : See-saw
(i) One lone pair
(ii) Four bond pair
(A) BrF5

Structure : Square pyramidal
(i) One lone pair
(ii) Four bond pair
(B) ClF3
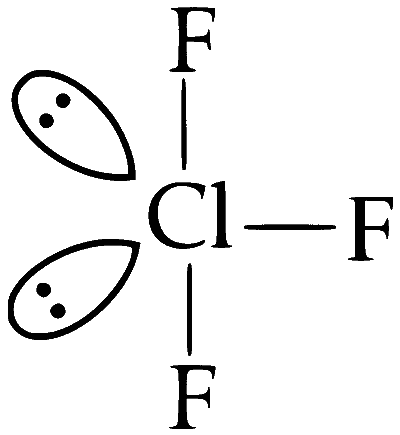
Structure : T-Shaped
(i) Two lone pair
(ii) Three bond pair
(C) XeF4

Structure : Square planar
(i) Two lone pair
(ii) Four bond pair
(D) SF4

Structure : See-saw
(i) One lone pair
(ii) Four bond pair
Comments (0)


