JEE Advance - Chemistry (2016 - Paper 1 Offline - No. 2)
According to the Arrhenius equation,
a high activation energy usually implies a fast reaction
rate constant increases with increase in temperature. This is due to a greater number of collisions whose energy exceeds the activation energy
higher the magnitude of activation energy, stronger is the temperature dependence of the rate constant.
the pre-exponential factor is a measure of the rate at which collisions occur, irrespective of their
energy.
Explanation
The Arrhenius equation is a mathematical model of the temperature dependence of reaction rates. It is expressed as follows:
$$ k=\mathrm{Ae}^{-\mathrm{E}_a / \mathrm{RT}} $$
where :
- k is the rate constant
- A is the pre-exponential factor (also known as the frequency factor)
- Ea is the activation energy
- R is the universal gas constant
- T is the temperature (in Kelvin)
- e is Euler's number, a mathematical constant approximately equal to 2.71828
The options provided correlate to the Arrhenius equation as follows:
Option A : Incorrect. A high activation energy usually implies a slower reaction, not a fast one. The activation energy is the energy barrier that needs to be overcome for a reaction to occur. The higher the activation energy, the fewer the number of molecules that have enough energy to surpass this barrier and react, resulting in a slower reaction rate.
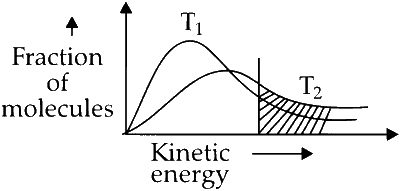
Option B : Correct. Temperature $\mathrm{T}_2$ is greater than $\mathrm{T}_1$; hence, the fraction of molecules (shaded in green) having energy greater than activation energy increases with increase in temperature result, rate of reaction increases.
Higher temperature, more number of collisions (between extents) having energy greater than activation energy happen increases speed of reaction.
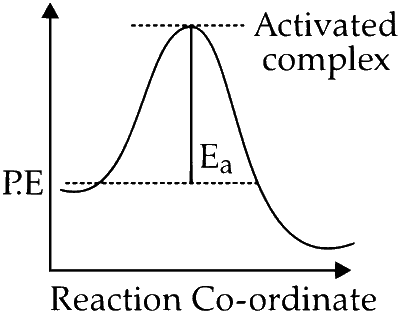
Option C : Correct. A reaction at higher activation energy makes the reaction rate slow. An increase in temperature causes reactant molecules to cross the high energy barrier; i.e., $E_a$ and form activated complex.
Option D : Correct. The pre-exponential factor (A) takes into account the probability of proper orientation of reactant molecules to form the product and the frequency at which reactant molecules collide to form the product.
$$ \mathrm{A}=\mathrm{Z}_{\mathrm{AB}} \times \mathrm{P} $$
$Z_{A B}$ : Collision frequency or frequency with which A collides with B.
P : Probability of reactant molecules oriented in right orientation with respect to each other to form the product.
Option (D), where pre-exponential factor affects the rate reaction irrespective of the energy they possess.
$$ k=\mathrm{Ae}^{-\mathrm{E}_a / \mathrm{RT}} $$
where :
- k is the rate constant
- A is the pre-exponential factor (also known as the frequency factor)
- Ea is the activation energy
- R is the universal gas constant
- T is the temperature (in Kelvin)
- e is Euler's number, a mathematical constant approximately equal to 2.71828
The options provided correlate to the Arrhenius equation as follows:
Option A : Incorrect. A high activation energy usually implies a slower reaction, not a fast one. The activation energy is the energy barrier that needs to be overcome for a reaction to occur. The higher the activation energy, the fewer the number of molecules that have enough energy to surpass this barrier and react, resulting in a slower reaction rate.

Option B : Correct. Temperature $\mathrm{T}_2$ is greater than $\mathrm{T}_1$; hence, the fraction of molecules (shaded in green) having energy greater than activation energy increases with increase in temperature result, rate of reaction increases.
Higher temperature, more number of collisions (between extents) having energy greater than activation energy happen increases speed of reaction.

Option C : Correct. A reaction at higher activation energy makes the reaction rate slow. An increase in temperature causes reactant molecules to cross the high energy barrier; i.e., $E_a$ and form activated complex.
Option D : Correct. The pre-exponential factor (A) takes into account the probability of proper orientation of reactant molecules to form the product and the frequency at which reactant molecules collide to form the product.
$$ \mathrm{A}=\mathrm{Z}_{\mathrm{AB}} \times \mathrm{P} $$
$Z_{A B}$ : Collision frequency or frequency with which A collides with B.
P : Probability of reactant molecules oriented in right orientation with respect to each other to form the product.
Option (D), where pre-exponential factor affects the rate reaction irrespective of the energy they possess.
Comments (0)


