JEE Advance - Chemistry (2016 - Paper 1 Offline - No. 18)
The correct statements about of the following reaction sequence is (are)
Cumene (C9H12) $$\mathrel{\mathop{\kern0pt\longrightarrow}
\limits_{(ii)\,{H_3}{O^ + }}^{(i)\,{O_2}}} $$ P $$\mathrel{\mathop{\kern0pt\longrightarrow}
\limits_{}^{CHC{l_3}/NaOH}} $$ Q (major) + R (minor)
Q $$\mathrel{\mathop{\kern0pt\longrightarrow}
\limits_{PhC{H_2}Br}^{NaOH}} $$ S
R is steam volatile.
Q gives dark violet coloration with 1% aqueous FeCl3 solution.
S gives yellow precipitate with 2, 4-dinitrophenylhydrazine.
S gives dark violet coloration with 1% aqueous FeCl3 solution.
Explanation
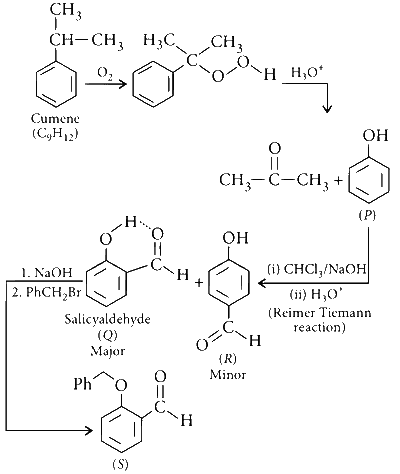
'Q' is steam volatile due to intramolecular hydrogen bonding while 'R' undergoes intermolecular hydrogen bonding hence, has higher boiling point. 'Q' gives dark violet coloration with 1% aqueous FeCl3 solution due to the presence of phenolic group while 'S' gives yellow precipitate with 2, 4-dinitrophenyl hydrazine due to the presence of aldehydic group ($$-$$CHO).
Comments (0)


