JEE Advance - Chemistry (2016 - Paper 1 Offline - No. 17)
Among [Ni(CO)4], [NiCl4]2$$-$$, [Co(NH3)4)Cl2]Cl, Na3[CoF6], Na2O2 and CsO2, the total number of paramagnetic compound is
2
3
4
5
Explanation
For the given complexes:
In $\left[\mathrm{Ni}(\mathrm{CO})_4\right]$ : $\mathrm{Ni}$ (at. no. 28): $1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 3 d^8 4 s^2$
$\mathrm{CO}$ being a strong field ligand causes the electrons to pair; hence, the complex diamagnetic.
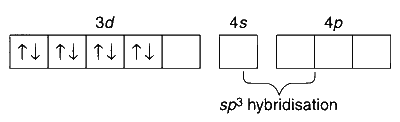
$$ \text {In }\left[\mathrm{NiCl}_4\right]^{2-}: \mathrm{Ni}^{2+} \text { (at. No. 26): } 1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 3 d^8 $$
There are two unpaired electrons, so the complex is paramagnetic.
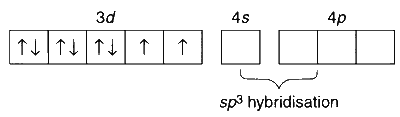
$$ \begin{aligned} & \mathrm{In}\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_4 \mathrm{Cl}_2\right] \mathrm{Cl}: \mathrm{Co}^{3+}\text {(at. No. 24): } 1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 3 d^6 \end{aligned} $$
The ligand $\mathrm{NH}_3$ results in formation of low spin complex, which is diamagnetic.
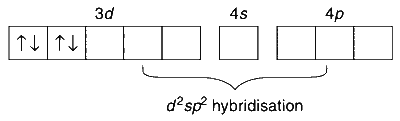
$$ \text {In } \mathrm{Na}_3\left[\mathrm{CoF}_6\right]: \mathrm{Co}^{3+}\text {(at. No. 24): } 1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 3 d^6 $$
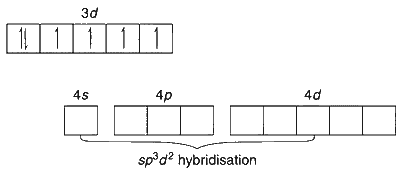
$\mathrm{F}$ is a weak field ligand and results in the formation of high spin complex with four unpaired electrons, which is paramagnetic.
In $\mathrm{Na}_2 \mathrm{O}_2$ : The molecular orbital configuration of $\mathrm{O}_2^{2-}$ is
$$ \sigma 1 s^2, \sigma^* 1 \mathrm{~s}^2, \sigma 2 s^2, \sigma^* 2 s^2, \sigma 2 p_z^2,\left\{\begin{array}{l} \pi 2 p_x^2 \\ \pi 2 p_y^2 \end{array},\left\{\begin{array}{l} \pi * 2 p_x^2 \\ \pi * 2 p_y^2 \end{array}\right.\right. $$
There is no unpaired electron, so it is diamagnetic.
In $\mathrm{CsO}_2$ : The molecular orbital configuration of $\mathrm{O}_2^{-}$is
$$ \sigma 1 s^2, \sigma * 1 \mathrm{~s}^2, \sigma 2 s^2, \sigma * 2 s^2, \sigma 2 p_z^2,\left\{\begin{array}{l} \pi 2 p_x^2 \\ \pi 2 p_y^2 \end{array},\left\{\begin{array}{l} \pi * 2 p_x^2 \\ \pi * 2 p_y^1 \end{array}\right.\right. $$
There is one unpaired electron, so it is paramagnetic. Hence, there are three paramagnetic compounds.
In $\left[\mathrm{Ni}(\mathrm{CO})_4\right]$ : $\mathrm{Ni}$ (at. no. 28): $1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 3 d^8 4 s^2$
$\mathrm{CO}$ being a strong field ligand causes the electrons to pair; hence, the complex diamagnetic.

$$ \text {In }\left[\mathrm{NiCl}_4\right]^{2-}: \mathrm{Ni}^{2+} \text { (at. No. 26): } 1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 3 d^8 $$
There are two unpaired electrons, so the complex is paramagnetic.

$$ \begin{aligned} & \mathrm{In}\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_4 \mathrm{Cl}_2\right] \mathrm{Cl}: \mathrm{Co}^{3+}\text {(at. No. 24): } 1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 3 d^6 \end{aligned} $$

The ligand $\mathrm{NH}_3$ results in formation of low spin complex, which is diamagnetic.
$$ \text {In } \mathrm{Na}_3\left[\mathrm{CoF}_6\right]: \mathrm{Co}^{3+}\text {(at. No. 24): } 1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 3 d^6 $$

$\mathrm{F}$ is a weak field ligand and results in the formation of high spin complex with four unpaired electrons, which is paramagnetic.
In $\mathrm{Na}_2 \mathrm{O}_2$ : The molecular orbital configuration of $\mathrm{O}_2^{2-}$ is
$$ \sigma 1 s^2, \sigma^* 1 \mathrm{~s}^2, \sigma 2 s^2, \sigma^* 2 s^2, \sigma 2 p_z^2,\left\{\begin{array}{l} \pi 2 p_x^2 \\ \pi 2 p_y^2 \end{array},\left\{\begin{array}{l} \pi * 2 p_x^2 \\ \pi * 2 p_y^2 \end{array}\right.\right. $$
There is no unpaired electron, so it is diamagnetic.
In $\mathrm{CsO}_2$ : The molecular orbital configuration of $\mathrm{O}_2^{-}$is
$$ \sigma 1 s^2, \sigma * 1 \mathrm{~s}^2, \sigma 2 s^2, \sigma * 2 s^2, \sigma 2 p_z^2,\left\{\begin{array}{l} \pi 2 p_x^2 \\ \pi 2 p_y^2 \end{array},\left\{\begin{array}{l} \pi * 2 p_x^2 \\ \pi * 2 p_y^1 \end{array}\right.\right. $$
There is one unpaired electron, so it is paramagnetic. Hence, there are three paramagnetic compounds.
Comments (0)


