JEE Advance - Chemistry (2016 - Paper 1 Offline - No. 1)
A plot of the number of neutrons (N) against the number of protons (P) of stable nuclei exhibits upward
deviation from linearity for atomic number, Z > 20. For an unstable nucleus having N/P ratio less than 1,
the possible mode(s) of decay is(are)
$$\beta ^-$$-decay ( $$\beta$$ emission)
orbital or K-electron capture
neutron emission
$$\beta ^+$$-decay (positron emission)
Explanation
Plot of Z (number of protons) verses N (number
of neutrons) is represented as :
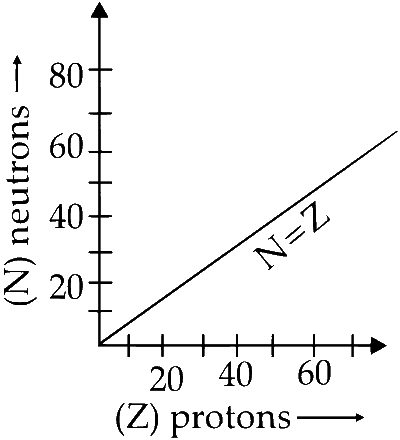
(a) For a stable nucleus $\mathrm{N} / \mathrm{Z}\left(\frac{\text { no. of neutrons }}{\text { no. of protons }}\right)$ should be equal to 1.
This happens for elements till atomic number $(Z=20)$. For such nucleus number of proton are equal to number of neutrons.
(b) For heavier nucleides with atomic number $(\mathrm{Z})$ greater than $20(Z>20)$, the number of neutron are more than proton as seen by curved (green) appearance in the plot.
(c) For nucleids with $\mathrm{N} / \mathrm{Z}<1$, the number of neutrons are less than the protons making the nucleus unstable. This is due to increase in coulombic repulsions between positively charged protons.
Such nuclei become stable by K-electron capture and positron emission.
K-electron capture :
$$ { }_1^1 p+{ }_{-1}^0 e \rightarrow{ }_0^1 n $$
The K-electron capture, proton is converted to the neutron as increases the $\mathrm{N} / \mathrm{Z}$ ratio.
$\beta^{ \pm}$decay (positron emission) :
$$ { }_1^1 p \rightarrow{ }\underset{neutron}{_0^1 n}+\underset{positron}{e^{+}}+\underset{electron \,nutrino}{v_e} $$
Proton decays to a neutron, positron and electron nutrino. This decreases the number of protons and increases the number of neutrons, thereby increasing $\mathrm{N} / \mathrm{Z}$ ratio.
Options (B) and (D) are correct.

(a) For a stable nucleus $\mathrm{N} / \mathrm{Z}\left(\frac{\text { no. of neutrons }}{\text { no. of protons }}\right)$ should be equal to 1.
This happens for elements till atomic number $(Z=20)$. For such nucleus number of proton are equal to number of neutrons.
(b) For heavier nucleides with atomic number $(\mathrm{Z})$ greater than $20(Z>20)$, the number of neutron are more than proton as seen by curved (green) appearance in the plot.
(c) For nucleids with $\mathrm{N} / \mathrm{Z}<1$, the number of neutrons are less than the protons making the nucleus unstable. This is due to increase in coulombic repulsions between positively charged protons.
Such nuclei become stable by K-electron capture and positron emission.
K-electron capture :
$$ { }_1^1 p+{ }_{-1}^0 e \rightarrow{ }_0^1 n $$
The K-electron capture, proton is converted to the neutron as increases the $\mathrm{N} / \mathrm{Z}$ ratio.
$\beta^{ \pm}$decay (positron emission) :
$$ { }_1^1 p \rightarrow{ }\underset{neutron}{_0^1 n}+\underset{positron}{e^{+}}+\underset{electron \,nutrino}{v_e} $$
Proton decays to a neutron, positron and electron nutrino. This decreases the number of protons and increases the number of neutrons, thereby increasing $\mathrm{N} / \mathrm{Z}$ ratio.
Options (B) and (D) are correct.
Comments (0)


