JEE Advance - Chemistry (2015 - Paper 1 Offline - No. 8)
Not considering the electronic spin, the degeneracy of the second excited state( n = 3) of H atom is 9, while
the degeneracy of the second excited state of H– is ___________.
Answer
3
Explanation
(i) Number of electrons in hydride ion $\left(\mathrm{H}^{-}\right)$ is $=2$
(ii) Electronic configuration of ground state in $\mathrm{H}^{-}$ ion (G.S) = 1s2
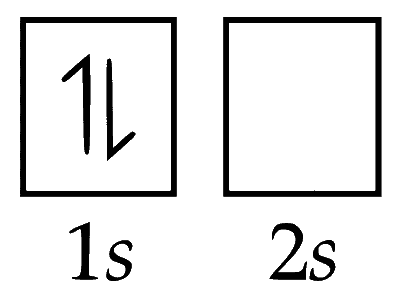
(iii) Electronic configuration of first excited state of $\mathrm{H}^{-}$ion $\left(\mathrm{E}{S_1}\right)$
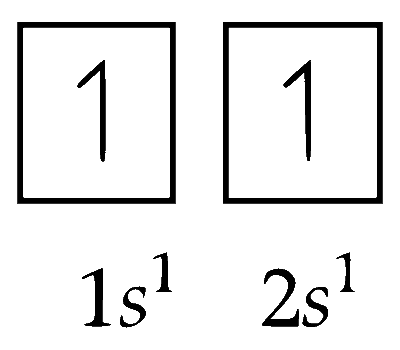
(iv) Electronic configuration of second excited state of $\mathrm{H}^{-}\left(\right.$E.S $\left._2\right)$
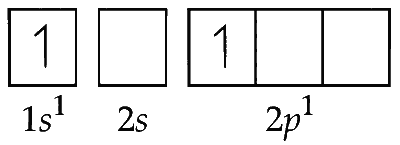
(v) The electron in $2 p$ orbital can occupy any three $2 p$ orbitals $2 p_{x^{\prime}} 2 p_y$ and $2 p_z$ as follows :
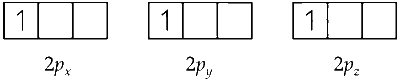
Hence, three degenerate orbitals represents second excited state of H–.
Comments (0)


