JEE Advance - Chemistry (2015 - Paper 1 Offline - No. 7)
Among the triatomic molecules/ions, BeCl2, $$N_3^-$$, N2O, $$NO_2^+$$, O3, SCl2, $$ICl_2^-$$, $$I_3^-$$ and XeF2, the total number of linear molecule(s)/ion(s) where the hybridization of the central atom does not have contribution from the d-orbital(s) is
[Atomic number: S = 16, Cl = 17, I = 53 and Xe = 54]
[Atomic number: S = 16, Cl = 17, I = 53 and Xe = 54]
Answer
4
Explanation
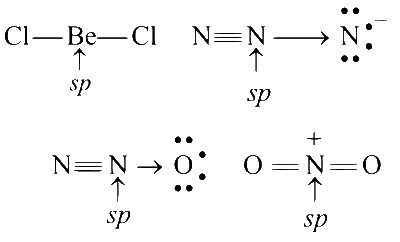
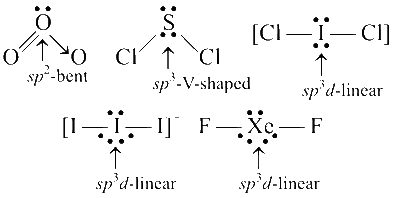
Comments (0)


