JEE Advance - Chemistry (2015 - Paper 1 Offline - No. 20)
The % yield of ammonia as a function of time in the reaction
N2(g) + 3H2(g) $$\rightleftharpoons$$ 2NH3(g), $$\Delta$$H < 0 at (P, T1) is given below:
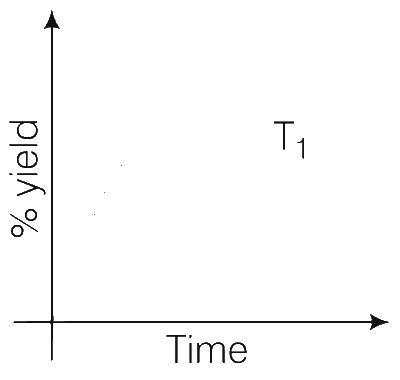
If this reactions is conducted at (P, T2), with T2 > T1, the % yield of ammonia as a function of time is represented by




Explanation
The equilibrium yield of ammonia gets lowered with the increase of temperature. However, at higher temperature the initial rate of forward reaction would be greater than at lower temperature that is why the percentage yield of NH3 would be more initially.
Comments (0)


