JEE Advance - Chemistry (2015 - Paper 1 Offline - No. 12)
For the octahedral complexes of Fe3+ in SCN$$-$$ (thiocyana-to-S) and in CN$$-$$ ligand environments, the difference between the spin-only magnetic moments in Bohr magnetons (when approximated to the nearest integer) is __________.
[Atomic number Fe = 26]
Answer
4
Explanation
The spin only magnetic moment is given by $$\mu = \sqrt {n(n + 1)} $$, where n is the number of unpaired electrons.
Fe3+ complex with weak field ligand SCN$$-$$ contains five unpaired electrons.
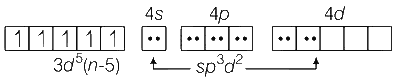
Therefore, $$\mu$$ = 5.9 BM.
Fe3+ complex with strong field ligand CN$$-$$ contains one unpaired electron.
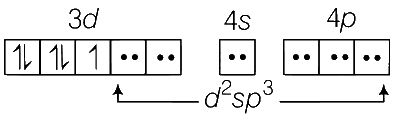
Therefore, $$\mu$$ = 1.73 BM.
Thus, the difference in spin only magnetic moment is $$\approx$$ 4.
Comments (0)


