JEE Advance - Chemistry (2015 - Paper 1 Offline - No. 11)
The number of lone pairs of electrons in N2O3 is ___________.
Answer
8
Explanation
The structure of N2O3 is
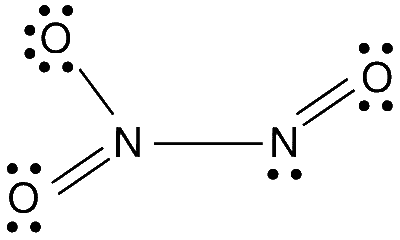
Therefore, the total number of lone pairs is 8.
Comments (0)

The structure of N2O3 is

Therefore, the total number of lone pairs is 8.
