JEE Advance - Chemistry (2014 - Paper 2 Offline - No. 6)
Isomers of hexane, based on their branching, can be divided into three distinct classes as shown in the figure.
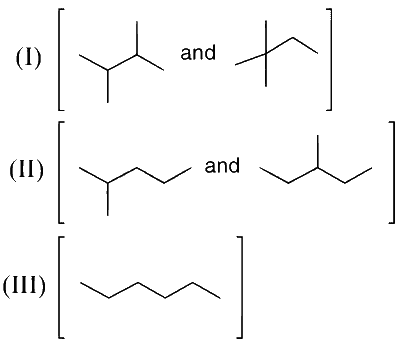
The correct order of their boiling point is
I > II > III
III > II > I
II > III > I
III > I > III
Explanation
As the branching increases, the boiling point decreases. This is because the van der Waals forces of attraction decrease.
Comments (0)


