JEE Advance - Chemistry (2014 - Paper 2 Offline - No. 16)
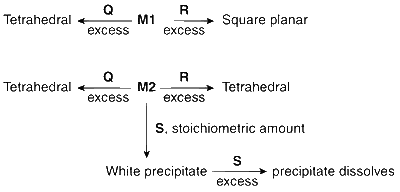
Reagent S is
K4[Fe(CN)6]
Na2HPO4
K2CrO4
KOH
Explanation
The reaction involved is
Zn2+ + OH$$-$$ $$\to$$ Zn(OH)2 $$\buildrel {O{H^ - }} \over \longrightarrow $$ [Zn(OH)4]2$$-$$ (soluble)
Comments (0)