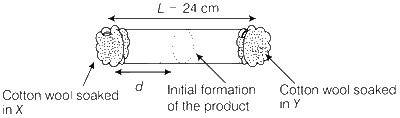
JEE Advance - Chemistry (2014 - Paper 2 Offline - No. 12)
The experimental value of d is found to be smaller than the estimate obtained using Graham's law. This is due to
larger mean free path for X as compared to that of Y.
larger mean free path for Y as compared to that of X.
increased collision frequency of Y with the inert gas as compared to that of X with the inert gas.
increased collision frequency of X with the inert gas as compared to that of Y with the inert gas.
Explanation
Collision frequency of X is greater than Y.
Comments (0)