JEE Advance - Chemistry (2014 - Paper 1 Offline - No. 9)
The pair(s) of reagents that yield paramagnetic species is/are
Na and excess of NH3
K and excess of O2
Cu and dilute HNO3
O2 and 2-ethylanthraquinol
Explanation
Reaction of alkali metals with ammonia depends upon the physical state of ammonia whether it is in gaseous state or liquid state. If ammonia is considered as a gas then reaction will be
(a) $$Na + \mathop {N{H_3}}\limits_{(Excess)} \to NaN{H_2} + {1 \over 2}{H_2}$$
(NaNH2 + 1/2H2 are diamagnetic) If ammonia is considered as a liquid then reaction will be
$$M + (x + y)N{H_3} \to {[M{(N{H_3})_x}]^ + } + {[e{(N{H_3})_y}]^ - }$$
$$\bullet$$ ammoniated electron
$$\bullet$$ blue colour
$$\bullet$$ paramagnetic
$$\bullet$$ very strong reducing agent
(b) $$K + \mathop {{O_2}}\limits_{(Excess)} \to \mathop {K{O_2}({K^ + },O_2^ - )}\limits_{Potassium\,\sup eroxide\,Paramagnetic} $$
(c) $$3Cu + 8HN{O_3} \to \mathop {3Cu{{(N{O_3})}_2}}\limits_{Paramagnetic} + \mathop {2NO}\limits_{Paramagnetic} + 4{H_2}O$$
(d)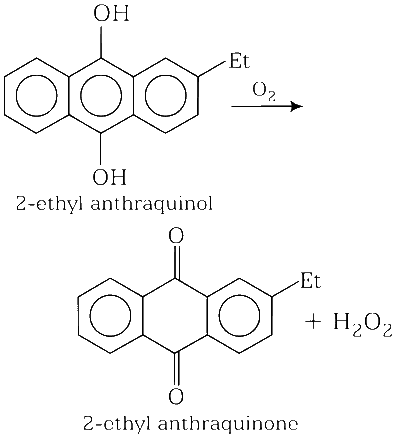
Hence, option (a), (b) and (c) are correct choices.
(a) $$Na + \mathop {N{H_3}}\limits_{(Excess)} \to NaN{H_2} + {1 \over 2}{H_2}$$
(NaNH2 + 1/2H2 are diamagnetic) If ammonia is considered as a liquid then reaction will be
$$M + (x + y)N{H_3} \to {[M{(N{H_3})_x}]^ + } + {[e{(N{H_3})_y}]^ - }$$
$$\bullet$$ ammoniated electron
$$\bullet$$ blue colour
$$\bullet$$ paramagnetic
$$\bullet$$ very strong reducing agent
(b) $$K + \mathop {{O_2}}\limits_{(Excess)} \to \mathop {K{O_2}({K^ + },O_2^ - )}\limits_{Potassium\,\sup eroxide\,Paramagnetic} $$
(c) $$3Cu + 8HN{O_3} \to \mathop {3Cu{{(N{O_3})}_2}}\limits_{Paramagnetic} + \mathop {2NO}\limits_{Paramagnetic} + 4{H_2}O$$
(d)

Hence, option (a), (b) and (c) are correct choices.
Comments (0)


