JEE Advance - Chemistry (2014 - Paper 1 Offline - No. 8)
For the reaction,
$${I^ - } + ClO_3^ - + {H_2}S{O_4} \to C{l^ - } + HSO_4^ - + {I_2}$$
the correct statement(s) in the balanced equation is/are
$${I^ - } + ClO_3^ - + {H_2}S{O_4} \to C{l^ - } + HSO_4^ - + {I_2}$$
the correct statement(s) in the balanced equation is/are
stoichiometric coefficient of HSO$$_4^ - $$ is 6
iodide is oxidised
sulphur is reduced
H2O is one of the products
Explanation
Oxidation half-reaction,
$$2{I^ - } \to {I_2} + 2{e^ - }$$ .... (i)
Here, I$$-$$ is converted into I2. Oxidation number of I is increasing from $$-$$1 to 0 hence, this is a type of oxidation reaction.
Reduction half-reaction
$$6{H^ + } + ClO_3^ - + 6{e^ - } \to C{l^ - } + 3{H_2}O$$ ..... (ii)
Here, H2O releases as a product. Hence, option (d) is correct.
Multiplying equation (i) by 3 and adding in equation (ii)
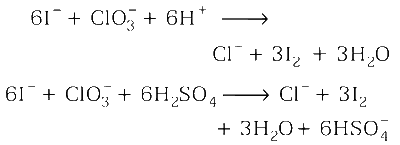
Stoichiometric coefficient of $$HSO_4^ - $$ is 6.
Hence, option (a), (b) and (d) are correct.
$$2{I^ - } \to {I_2} + 2{e^ - }$$ .... (i)
Here, I$$-$$ is converted into I2. Oxidation number of I is increasing from $$-$$1 to 0 hence, this is a type of oxidation reaction.
Reduction half-reaction
$$6{H^ + } + ClO_3^ - + 6{e^ - } \to C{l^ - } + 3{H_2}O$$ ..... (ii)
Here, H2O releases as a product. Hence, option (d) is correct.
Multiplying equation (i) by 3 and adding in equation (ii)
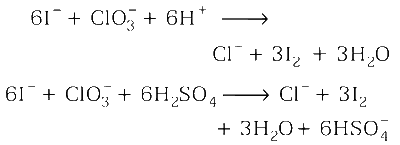
Stoichiometric coefficient of $$HSO_4^ - $$ is 6.
Hence, option (a), (b) and (d) are correct.
Comments (0)


