JEE Advance - Chemistry (2014 - Paper 1 Offline - No. 5)
A list of species having the formula XZ4 is given below.
XeF4, SF4 ,SiF4, $$BF_4^-$$, $$BrF_4^-$$, [Cu(NH3)4]2+, [FeCl4]2-, [CoCl4]2- and [PtCl4]2-
Defining shape on the basis of the location of X and Z atoms, the total number of species having a square planar shape is
XeF4, SF4 ,SiF4, $$BF_4^-$$, $$BrF_4^-$$, [Cu(NH3)4]2+, [FeCl4]2-, [CoCl4]2- and [PtCl4]2-
Defining shape on the basis of the location of X and Z atoms, the total number of species having a square planar shape is
Answer
4
Explanation
$\mathrm{XeF}_4, \mathrm{BrF}_4^{-},\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right]^{2+},\left[\mathrm{PtCl}_4\right]^{2-}$ are square planar as shown below :

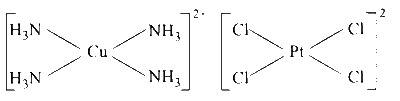
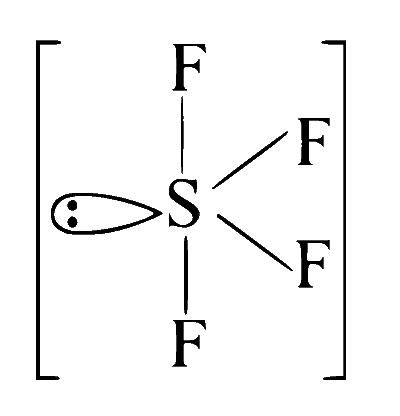
$\mathrm{SF}_4(\mathrm{See}-\mathrm{saw})$ as shown below:
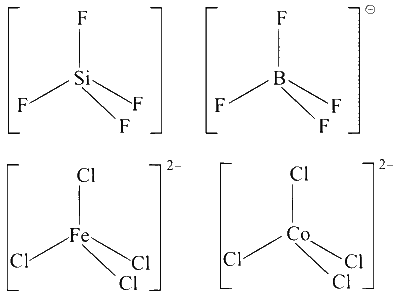
$\mathrm{SiF}_4, \mathrm{BF}_4^{-},\left[\mathrm{FeCl}_4\right]^{2-},\left[\mathrm{CoCl}_4\right]^{2-}$ are tetrahedral as shown below :

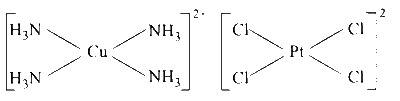
$\mathrm{SF}_4(\mathrm{See}-\mathrm{saw})$ as shown below:

$\mathrm{SiF}_4, \mathrm{BF}_4^{-},\left[\mathrm{FeCl}_4\right]^{2-},\left[\mathrm{CoCl}_4\right]^{2-}$ are tetrahedral as shown below :

Comments (0)


