JEE Advance - Chemistry (2014 - Paper 1 Offline - No. 18)
The total number(s) of stable conformers with non-zero dipole moment for the following compound is(are)
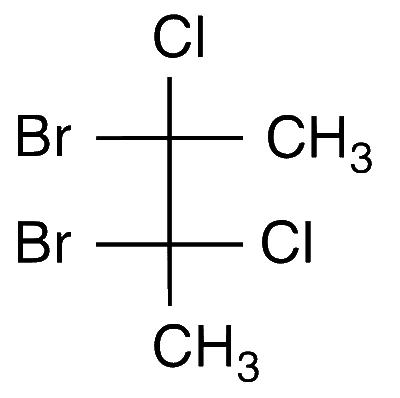

Answer
3
Explanation
The conformations of the given compound are as follows
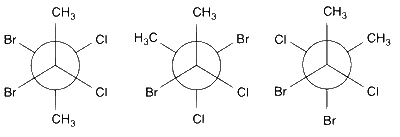
These three have non-zero dipole moment due to non-cancellation of all dipole moment created by C$$-$$Cl and C$$-$$Br bond.

These three have non-zero dipole moment due to non-cancellation of all dipole moment created by C$$-$$Cl and C$$-$$Br bond.
Comments (0)


