JEE Advance - Chemistry (2014 - Paper 1 Offline - No. 14)
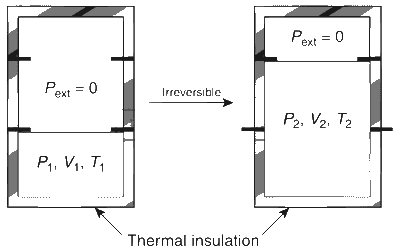
An ideal gas in thermally insulated vessel at internal pressure = p1, volume = V1 and absolute temperature = T1 expands irreversibly against zero external pressure, as shown in the diagram.
The final internal pressure, volume and absolute temperature of the gas are p2, V2 and T2, respectively. For this expansion
q = 0
T2 = T1
p2V2 = p1V1
p2V$$_2^\gamma $$ = p1$$_1^\gamma $$
Explanation
As the vessel is thermally insulated (so, q = 0). Therefore,
$$\Delta$$U = 0, $$\Delta$$T = 0 $$\Rightarrow$$ T1 = T2
According to combined gas law equation,
$${{{P_2}{V_2}} \over {{T_2}}} = {{{P_1}{V_1}} \over {{T_1}}} \Rightarrow {P_2}{V_2} = {P_1}{V_1}$$
$$\Delta$$U = 0, $$\Delta$$T = 0 $$\Rightarrow$$ T1 = T2
According to combined gas law equation,
$${{{P_2}{V_2}} \over {{T_2}}} = {{{P_1}{V_1}} \over {{T_1}}} \Rightarrow {P_2}{V_2} = {P_1}{V_1}$$
Comments (0)


