JEE Advance - Chemistry (2014 - Paper 1 Offline - No. 11)
Hydrogen bonding plays a central role in the following phenomena
ice floats in water
higher Lewis basicity of primary amines than tertiary amines in aqueous solutions
formic acid is more acidic than acetic acid
dimerisation of acetic acid in benzene
Explanation
(a) Ice floats in water due to the low density of ice as compare to water which is due to open cage like structure (formed by intermolecular H-bonding).
(b) Basic strength of RNH2 > R3N. It is also explained by hydrogen bonding.

(c)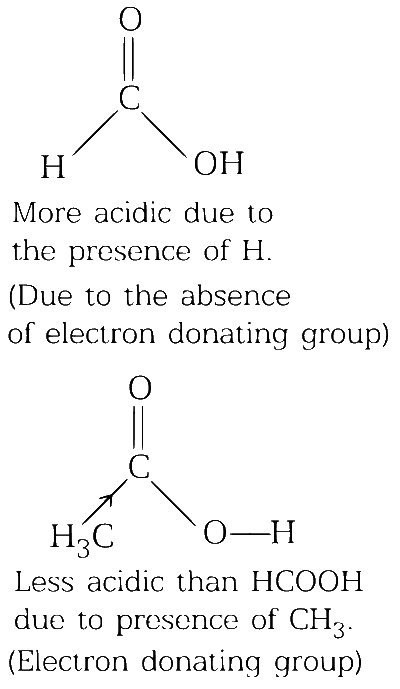
(d) Dimerisation of acetic acid in benzene is due to intermolecular hydrogen bonding.
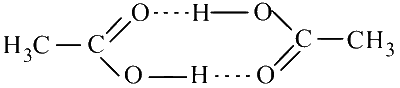
(b) Basic strength of RNH2 > R3N. It is also explained by hydrogen bonding.

(c)

(d) Dimerisation of acetic acid in benzene is due to intermolecular hydrogen bonding.
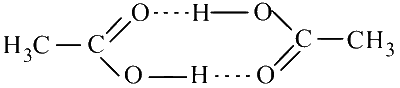
Comments (0)


