JEE Advance - Chemistry (2013 - Paper 1 Offline - No. 5)
Explanation
(i) The structure of tert-butyl cation $\left[\left(\mathrm{CH}_3\right)_3 \mathrm{C}^{\oplus}\right]$ is as follows :
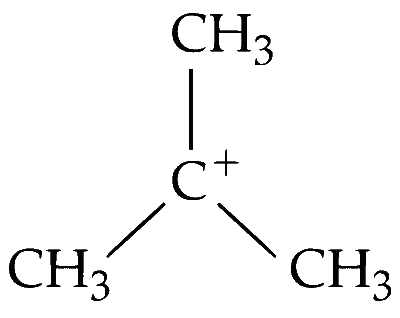
The carbocation is highly stable due to positive hyperconjugative $(+\mathrm{H})$ and inductive $(+\mathrm{I})$ effect.
(ii) The hyperconjugative effect is due to delocalication of $\sigma$ (sigma) electrons of $\mathrm{C}-\mathrm{H}$ sigma ( $\sigma$ ) bond on carbon adjacent to carbocation with an empty P orbital (on carbcation).
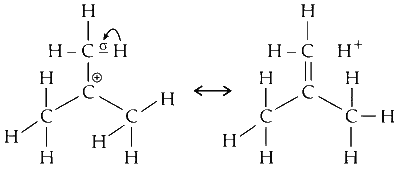
Hence, carbocation is stabilised due to formation of pi bond with adjacent carbon (due to delocalised) electrons of $\mathrm{C}-\mathrm{H}$ sigma bond). Its a s to p (empty) delocalisation.
(iii) There are eight more protons on carbons adjacent to carbocation. This gives eight more hyperconjugative structures. This impart stability to carbocation.
(iv) The structure of 2-butene is as follows :
$$
\mathrm{CH}_3-\mathrm{C}=\mathrm{C}-\mathrm{CH}_3
$$
The hyperconjugative effect is due to the delocalisation of sigma ( $\sigma$ ) electrons of $\mathrm{C}-\mathrm{H}$ bond with adjacent carbon containing pi bond.
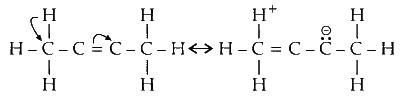
The electrons of $\mathrm{C}-\mathrm{H} $ $\sigma$ bond are donated to anti-bonding $\left(\pi^*\right)$ orbitals of carbon-carbon double bond. Its $\sigma $ $\mathrm{s}$ to $\pi^*$ delocalisation.
There are five more hydrogens from methyl groups attached to carbon-carbon double bond. Hence, five more hyperconjugative structures.
Comments (0)


