JEE Advance - Chemistry (2013 - Paper 1 Offline - No. 2)
The total number of lone-pairs of electrons in melamine is
Answer
6
Explanation
The structure of melamine is :
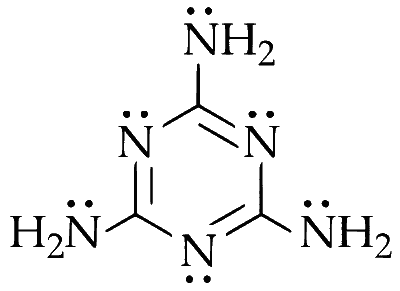
Each nitrogen has one lone pair of electrons. Number (no.) of nitrogen in a molecule $=6$
Total no. of lone pairs in melamine
$$ \begin{aligned} & =\text { No. of nitrogen } \times \text { lone pair } \\\\ & =6 \times 1=6 \end{aligned} $$
Hence, total number of lone pair on nitrogen is 6.
Comments (0)


