JEE Advance - Chemistry (2013 - Paper 1 Offline - No. 19)
EDTA4$$-$$ is ethylenediaminetetraacetate ion. The total number of N$$-$$Co$$-$$O bond angles in [Co(EDTA)]1$$-$$ complex ion is ________.
Answer
8
Explanation
EDTA is a multidentate ligand as it can donate six pairs of electrons – two pair from the two nitrogen atoms and four pair from the four terminal oxygens of the $$-$$COO- groups.
The structure of the complex is
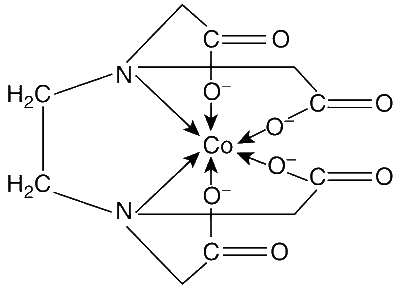
Therefore, the number of N$$-$$Co$$-$$O bonds are 8.
Comments (0)


